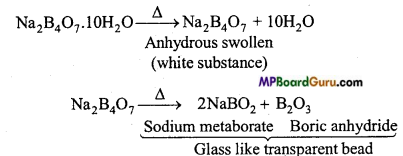
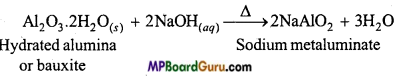
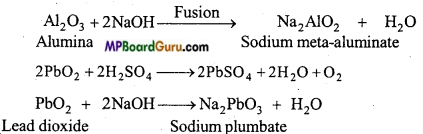
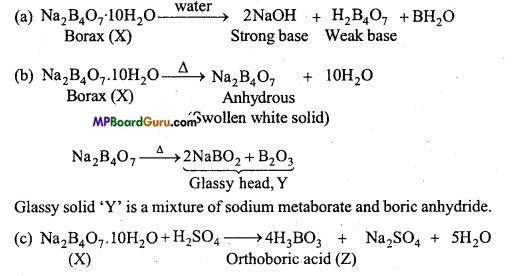
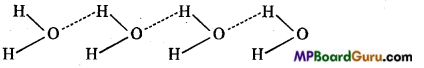
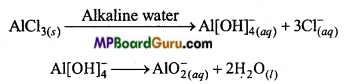
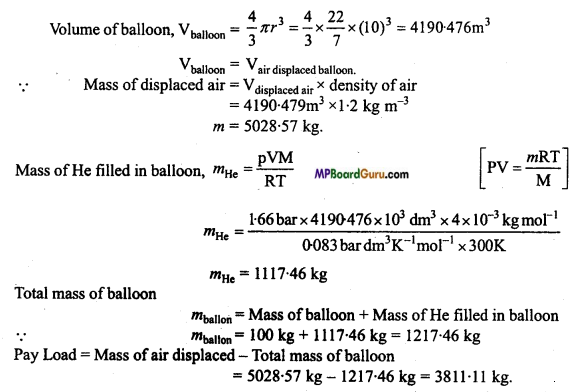
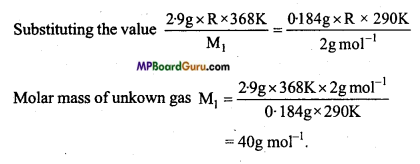
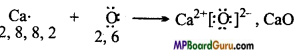
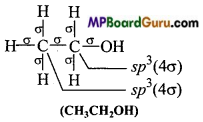
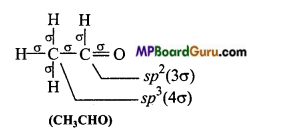
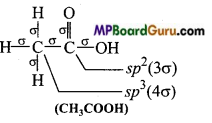
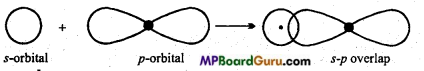
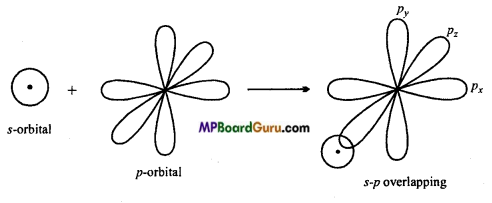
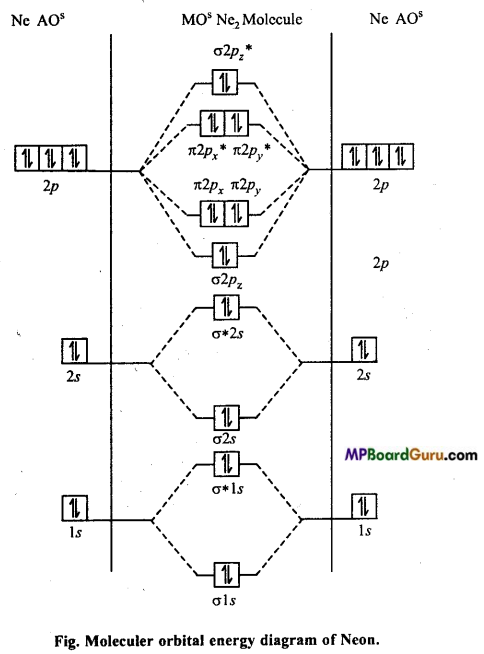
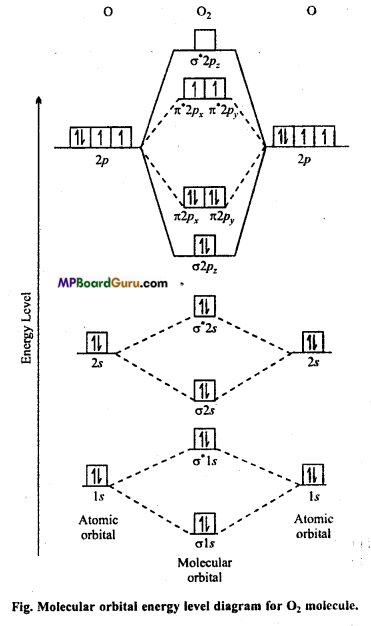
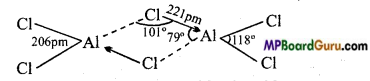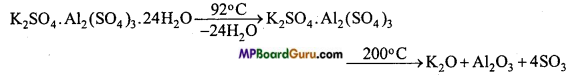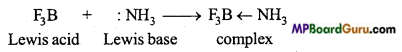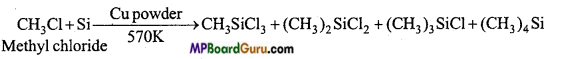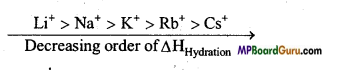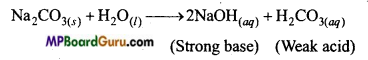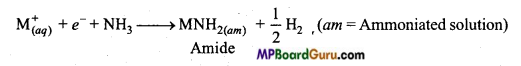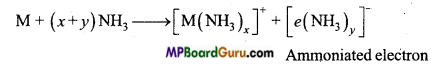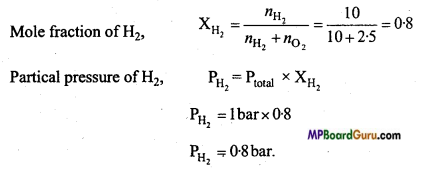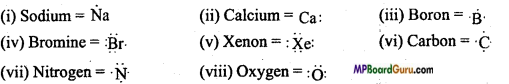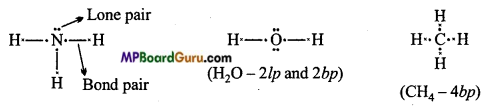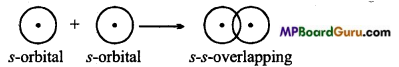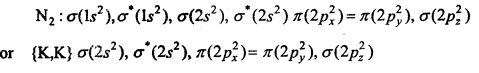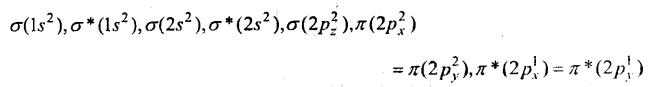MP Board Class 11th Chemistry Solutions Chapter 10 s-ब्लॉक तत्त्व
s-ब्लॉक तत्त्व NCERT अभ्यास प्रश्न
प्रश्न 1.
क्षार धातुओं के सामान्य भौतिक तथा रासायनिक गुण क्या हैं ?
उत्तर:
भौतिक गुण –
1. क्षार धातुएँ चाँदी के समान सफेद, नरम तथा हल्की धातुएँ होती हैं।
2. इनके धनत्व बहुत कम होते हैं (बड़े आकार के कारण)। यह वर्ग में नीचे जाने पर घटता है। यद्यपि पोटैशियम, सोडियम से हल्की धातु है।
3. क्षार धातुओं के गलनांक तथा क्वथनांक निम्न होते हैं, क्योंकि इन धातुओं में मात्र एक संयोजी इलेक्ट्रॉन के उपस्थिति के कारण इनके बीच दुर्बल धात्विक बंध होते हैं।
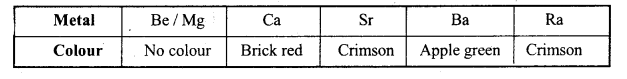
4. क्षार धातुएँ, Be तथा Mg इनके लवण ज्वाला में विशिष्ट रंग प्रदान करते हैं, क्योंकि इनके संयोजी इलेक्ट्रॉन आसानी से निम्न से उच्च ऊर्जा स्तर तक उत्तेजित हो जाते हैं –

रासायनिक गुण:
क्षार धातुएँ अपनी निम्न आयनन एन्थैल्पी के कारण अत्यधिक क्रियाशील होती है। इनकी क्रियाशीलता वर्ग में नीचे जाने पर घटती है –
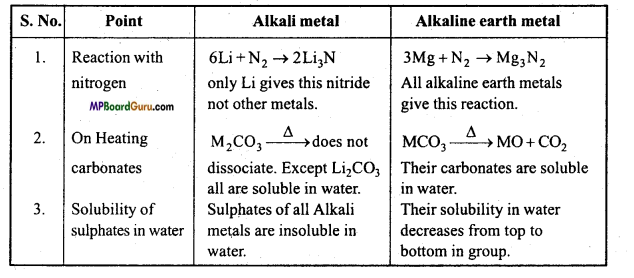
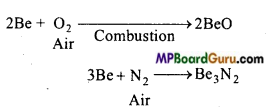
1. वायु के साथ अभिक्रियाशीलता:
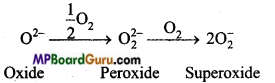
क्षार धातुएँ वायु की उपस्थिति में ऑक्साइड बनाने के कारण मलिन हो जाती है। लीथियम मोनोऑक्साइड, सोडियम परॉक्साइड तथा अन्य धातुएँ सुपर ऑक्साइड बनाती है।
- 4Li+ O2 → 2Li2O (ऑक्साइड)
- 2Na + O2 → NaO2 (परॉक्साइड)
- M+ O2 → MO2 (सुपरऑक्साइड) (M = K, Rb, Cs)
लीथियम वायु की नाइट्रोजन के साथ सीधे संयोग करके लीथियम नाइट्राइड (Li3N) बनाती है।
2. जल के साथ क्रिया:
क्षार धातुएँ जल के साथ क्रिया करके हाइड्रॉक्साइड तथा डाइहाइड्रोजन बनाती हैं।
2M + 2H2O → 2M+ + 2OH– + H2, (M = क्षार धातु) लीथियम का जल के साथ क्रिया सोडियम की तुलना में कम होता है। क्षार धातुएँ प्रोटॉन दाता स्पीशीज, जैसे – एल्कोहॉल, ऐल्काइन और गैसीय NHS से भी क्रिया करती हैं।
3. डाइहाइड्रोजन के साथ क्रिया:
क्षार धातुएँ डाइहाइड्रोजन के साथ सुगमतापूर्वक क्रिया करके आयनिक या लवणीय हाइड्राइड बनाती हैं।
![]()
(M = Li,Na, K आदि)
(iv) हैलोजन के साथ क्रिया:
क्षार धातुएँ हैलोजनों के साथ सुगमतापूर्वक क्रिया करके आयनिक हैलाइड बनाती हैं।
![]()
(M = क्षार धातु)
(v) अपचायक प्रकृति:
क्षार धातुएँ अपने संयोजी इलेक्ट्रॉन को सरलता से त्याग देती है। अतः ये प्रबल अपचायक होती हैं।
Na → Na+ +e–
लीथियम सर्वाधिक प्रबल तथा सोडियम दुर्बलतम अपचायक है।
(vi) दव अमोनिया में विलेयता:
सभी क्षार धातुएँ अमोनिया में विलेय हो जाती है तथा गहरे नीले रंग का विलयन बनाती है।
![]()
(vii) सभी क्षार धातुएँ परस्पर तथा अन्य धातुओं के साथ मिलकर मिश्रधातु बनाती हैं। मर्करी के साथ अमलगम बनाती है तथा इस प्रकार की क्रियाएँ अत्यधिक ऊष्माक्षेपी होती हैं।
प्रश्न 2.
क्षारीय मृदा धातुओं के सामान्य अभिलाक्षणिक गुणों में आवर्तिता की विवेचना कीजिए।
उत्तर:
वर्ग – 2 के तत्व Be, Mg, Ca, Sr, Ba तथा Ra को संयुक्त रूप से क्षारीय धातु कहते हैं (Be के अतिरिक्त)।
सामान्य गुण (A) परमाण्विक गुण:
1. इलेक्ट्रॉनिक विन्यास – इनके सामान्य इलेक्ट्रॉनिक विन्यास को [उत्कृष्ट गैस] ns2 द्वारा दर्शाते हैं।
2. परमाण्विक तथा आयनिक त्रिज्या – इन तत्वों की परमाण्विक तथा आयनिक त्रिज्याएँ एक ही आवर्त में निकटवर्ती क्षार धातुओं की अपेक्षा कम होती है। एक ही वर्ग में परमाण्विक तथा आयनिक त्रिज्याएँ, परमाणु संख्या में वृद्धि के साथ – साथ बढ़ती है।
3. आयनन एन्थैल्पी – क्षारीय मृदा धातुओं की प्रथम आयनन एन्थैल्पी, इनके छोटे आकार के कारण –
![]()
क्षारीय मृदा धातुओं की द्वितीय आयनन एन्थैल्पी, संगत क्षार धातुओं की अपेक्षा कम होती है।
4. जलयोजन एन्थैल्पी – क्षारीय मृदा धातुओं की जलयोजन एन्थैल्पी क्षार धातु आयनों के समान वर्ग में नीचे की ओर जाने से क्रमशः घटती है।
Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+
क्षारीय मृदा धातु आयनों की जलयोजन एन्थैल्पी, संगत क्षार धातुओं की अपेक्षा कम होती है।
(B) भौतिक गुण:
1. ये धातुएँ चाँदी के समान सफेद, चमकीली तथा नरम (परन्तु क्षार धातुओं की अपेक्षा कठोर) होती है।
2. इन धातुओं के छोटे आकार के कारण इनके गलनांक तथा क्वथनांक संगत क्षार धातुओं की अपेक्षा कम होते हैं। यह क्रम यद्यपि नियमित नहीं है।
3. ज्वाला के प्रति रंग – Be तथा Mg के अतिरिक्त सभी क्षारीय मृदा धातुएँ ज्वाला के साथ एक विशिष्ट रंग देते हैं। इनके विभिन्न रंग इलेक्ट्रॉनों को उत्तेजित तथा उत्तेजनहीन करने में प्रयुक्त ऊर्जाओं के विभिन्नता के कारण होता है।

4. घनत्व – इन धातुओं के घनत्व Be से Ca तक घटते हैं, तदुपरान्त बढ़ते हैं, ये धातुएँ अपने छोटे आकार के कारण, संगत क्षार धातुओं की अपेक्षा सघन, भारी तथा कठोर होती हैं।
(C) रासायनिक गुण:
क्षारीय मृदा धातुएँ क्षार धातुओं की अपेक्षा कम क्रियाशील होती हैं। इन धातुओं की क्रियाशीलता वर्ग में नीचे की ओर जाने पर क्रमशः घटती है।
1. वायु तथा जल के साथ क्रिया – Be तथा Mg वायु (ऑक्सीजन) तथा जल के प्रति अक्रिय होते हैं क्योंकि इनकी सतह पर ऑक्साइड की परत जम जाती है। यद्यपि, बेरीलियम चूर्ण वायु में आसानी से जल जाता है।
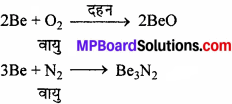
इसी प्रकार मैग्नीशियम अधिक विद्युत् धनात्मक है तथा वायु में चमकीले प्रकाश के साथ जलकर Mgo तथा Mg,N, बनाता है। Ca, Sr तथा Ba वायु के साथ शीघ्रता से क्रिया करके ऑक्साइड तथा नाइट्राइड बनाते हैं।
2. हाइड्रोजन के साथ क्रिया – Be के अतिरिक्त ये सभी धातुएँ गर्म करने पर हाइड्रोजन के साथ योग करके MH, प्रकार के हाइड्राइड बनाती है। (M = Be, Mg, Ca, Sr, Ba)
3. हैलोजन के साथ क्रिया – ये धातुएँ हैलोजन के साथ उच्च ताप पर क्रिया करके MX2 प्रकार के हैलाइड बनाती है। M + X2 → MX2,
(X = F, CI, Br)
4. अम्लों के साथ क्रिया-ये धातुएँ अम्लों के साथ शीघ्रता से क्रिया करती हैं तथा H, गैस मुक्त करती हैं।
M + 2HCl → MCl2 + H2
5. अपचायक प्रकृति- क्षार धातुओं की भाँति, क्षारीय मृदा धातुएँ भी प्रबल अपचायक प्रकृति की होती है। यद्यपि इनकी अपचायक क्षमता क्षार धातुओं की अपेक्षा कम होती है। वर्ग में नीचे जाने पर इनकी अपचायक क्षमता क्रमशः घटती है।
6. द्रव NH3 में विलेयता:
क्षार धातुओं की भाँति, क्षारीय मृदा धातुएँ द्रव अमोनिया में घुलकर गहरा नीला काला विलयन बनाती हैं।
M + (x + y)NH3 → [M(NH3)x]2+ + 2[e(NH3)y]–
प्रश्न 3.
क्षार धातुएँ प्रकृति में क्यों नहीं पाई जाती हैं ?
उत्तर:
क्षार धातुएँ अत्यधिक रासायनिक सक्रियता के कारण प्रकृति में मुक्त अवस्था में नहीं पाई जाती हैं। ये भूपर्पटी में हैलाइड, सल्फेट, कार्बोनेट, सिलिकेट, बोरेट, ऑक्साइड आदि अयस्कों के रूप में पाई जाती हैं।
प्रश्न 4.
Na2O2में सोडियम की ऑक्सीकरण अवस्था ज्ञात कीजिए।
उत्तर:
माना Na2O2में Na की ऑक्सीकरण अवस्था x है। H2O2 के समान Na2O2 में परॉक्साइड बंध (-O-O-) होता है। अत: Na2O2 में Na की ऑक्सीकरण अवस्था –
x × 2 + (-1) × 2 = 0
2x – 2 = 0
⇒ x = \(\frac { 2 }{ 2 }\) = +1
प्रश्न 5.
पोटैशियम की तुलना में सोडियम कम अभिक्रियाशील क्यों है ? बताइए। ‘
उत्तर:
पोटैशियम की आयनन एन्थैल्पी (∆iH) (419 kJmol-1) सोडियम की आयनन एन्थैल्पी (496 kJmol-1) की अपेक्षा कम होती है तथा पोटैशियम का मान इलेक्ट्रोड विभव, (-2.925 V), सोडियम के संगत मान (-2.714 V) की अपेक्षा अधिक ऋणात्मक है। यही कारण है कि पोटैशियम, सोडियम की अपेक्षा अधिक ऋणात्मक है। अत: पोटैशियम, सोडियम से अधिक क्रियाशील होता है।
प्रश्न 6.
निम्नलिखित के सन्दर्भ में क्षार धातुओं एवं क्षारीय मृदा धातुओं की तुलना कीजिए –
- आयनन एन्थैल्पी
- ऑक्साइडों की क्षारकता
- हाइड्रॉक्साइडों की विलेयता।
उत्तर:
- आयनन एन्थैल्पी – अधिक नाभिक आवेश एवं छोटे परमाणु आकार के कारण, क्षारीय मृदा धातुओं की आयनन एन्थैल्पी संगत क्षार धातुओं से अधिक होती है।
- ऑक्साइडों की क्षारकता – क्षार धातुओं के आयनन एन्थैल्पी कम अथवा वैद्युत धनात्मक गुण संगत क्षारीय मृदा धातुओं से अधिक होते हैं। अतः क्षारीय धातु ऑक्साइड संगत क्षारीय मृदा धातु ऑक्साइडों से अधिक क्षारीय होते हैं।
- हाइड्रॉक्साइडों की विलेयता – क्षारीय मृदा धातुओं के हाइड्रॉक्साइडों की विलेयता संगत क्षारीय हाइड्रॉक्साइडों की अपेक्षा कम होती है।
प्रश्न 7.
लीथियम किस प्रकार मैग्नीशियम से रासायनिक गुणों में समानताएँ दर्शाता है ?
उत्तर:
लीथियम तथा मैग्नीशियम समान आकार के कारण गुणों में अत्यधिक समानता प्रदर्शित करते हैं –
(परमाण्विक त्रिज्या : Li = 152pm, Mg = 160pm; आयनिक त्रिज्या Li+ = 76pm, Mg2+ = 72pm)। इनके समान गुण निम्न हैं –
1. लीथियम तथा मैग्नीशियम दोनों अपने संबंधित वर्गों के तत्वों की अपेक्षा हल्के तथा कठोर हैं।
2. लीथियम तथा मैग्नीशियम दोनों जल के साथ धीरे-धीरे क्रिया करते हैं।
3. लीथियम तथा मैग्नीशियम दोनों के ऑक्साइड तथा हाइड्रॉक्साइड जल में बहुत कम विलेय हैं। इनके हाइड्रॉक्साइड गर्म करने पर अपघटित होते हैं।
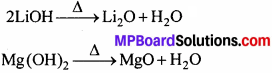
4. Li तथा Mg दोनों वायु की नाइट्रोजन से सीधे क्रिया करके नाइट्राइड (Li3N तथा Mg3N2) बनाते हैं।
5. Li तथा Mg दोनों गर्म करने पर कार्बन के साथ संयुक्त होते हैं।
- 2Li + 2C → Li2C2
- Mg + 2C → MgC2
6. Li एवं Mg दोनों का ऑक्सीजन में गर्म करने पर मोनोऑक्साइड बनाते हैं।
- 4Li + O2 → 2Li2o
- 2Mg + O2 → 2MgO
7. Li2SO4 , MgSO4 की भाँति फिटकरी नहीं बनाता है।
8. LiCl, MgCl दोनों प्रस्वेद्य है तथा जलीय विलयन में हाइड्रेट (LiCl – 2H2O तथा MgCl26H2O) के रूप में क्रिस्टलीय होते हैं।
9. LiCO3 तथा MgCO3 दोनों गर्म करने पर आसानी से अपघटित होकर ऑक्साइड तथा CO2 बनाते हैं।
10. Li तथा Mg दोनों द्वारा ठोस हाइड्रोजन कार्बोनेट नहीं बनाए जा सकते हैं।
11. LiNO3 तथा Mg(NO3)2 दोनों गर्म करने पर अपघटित होकर नाइट्रोजन डाइऑक्साइड बनाते हैं।
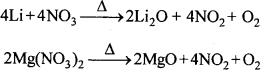
प्रश्न 8.
क्षार धातुएँ तथा क्षारीय मृदा धातुएँ रासायनिक अपचयन विधि से क्यों प्राप्त नहीं किए जा सकते ? समझाइए।
उत्तर:
- क्षार धातुएँ तथा क्षारीय मृदा धातुएँ स्वयं में प्रबल अपचायक हैं। अतः ये धातुएँ अपने ऑक्साइंड तथा अन्य यौगिकों द्वारा रासायनिक अपचयन विधि द्वारा प्राप्त नहीं की जा सकती है।
- इन धातुओं की प्रकृति अत्यधिक विद्युत् धनात्मक होती है। अतः इनके लवणों में जलीय विलयन से इन्हें अन्य धातुओं द्वारा विस्थापित नहीं किया जा सकता है।
प्रश्न 9.
प्रकाश वैद्युत सेल में लीथियम के स्थान पर पोटैशियम एवं सीज़ियम क्यों प्रयुक्त किए जाते हैं?
उत्तर:
पोटैशियम तथा सीज़ियम की आयनन एन्थैल्पी लीथियम की अपेक्षा अधिक कम होती है। अतः ये धातुएँ प्रकाश में रखने पर इलेक्ट्रॉन आसानी से उत्सर्जित करती है, परन्तु लीथियम ऐसा नहीं कर पाती है। यही कारण है Li की अपेक्षा K तथा Cs का प्रयोग प्रकाश वैद्युत सेल में किया जाता है।
प्रश्न 10.
जब एक क्षार धातु को दव अमोनिया में घोला जाता है, तब विलियन विभिन्न रंग प्राप्त कर सकता है। इस प्रकार के रंग-परिवर्तन कारण बताइए।
उत्तर:
क्षार धातुओं का अमोनिया में तनु विलयन का रंग गहरा नीला होता है क्योंकि अमोनीकृत इलेक्ट्रॉन प्रकाश के अदृश्य क्षेत्र में ऊर्जा अवशोषित करते हैं। यदि विलयन की सान्द्रता 3M से अधिक बढ़ा दी जाये तो रंग ताँबे – काँस्य जैसा हो जाता है।
प्रश्न 11.
ज्वाला को बेरीलियम एवं मैग्नीशियम कोई रंग प्रदान नहीं करते हैं, जबकि अन्य क्षारीय मृदा धातुएँ ऐसा करती हैं, क्यों?
उत्तर:
Be तथा Mg परमाणु अपने छोटे आकार तथा अधिक प्रभावी नाभिकीय आवेश के कारण अपने इलेक्ट्रॉनों को अधिक प्रबलता सं बाँधे रखते हैं। अतः इन्हें उच्च उत्तेजन ऊर्जा की आवश्यकता होती है तथा ये बुन्सन ज्वाला द्वारा उत्तेजित नहीं हो पाते हैं। जबकि अन्य क्षारीय मृदा धातुओं के उच्च ऊर्जा स्तरों में इलेक्ट्रॉनों का सरलतापूर्वक उत्सर्जन हो सकता है। अतः ज्वाला में विशिष्ट रंग देते हैं।
प्रश्न 12.
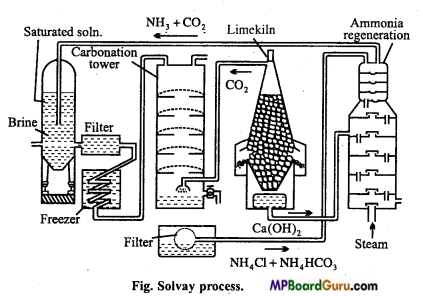
सॉल्वे प्रक्रम में होने वाली विभिन्न अभिक्रियाओं की विवेचना कीजिए।
उत्तर:
सॉल्वे अमोनिया प्रक्रम में, अमोनिया द्वारा संतृप्त ब्राइन (NaCl का सान्द्र विलयन) में CO2 प्रवाहित की जाती है। इस प्रक्रम में शीघ्र विलेय सोडियम बाइकार्बोनेट बनता है।
![]()
इस प्रकार बने सोडियम बाइकार्बोनेट को छानकर, सुखाकर तथा गर्म करके सोडियम कार्बोनेट प्राप्त करते हैं।
![]()
कार्बोनेटिंग स्तंभ में प्रयुक्त CO2 को कैल्सियम कार्बोनेट को गर्म करके प्राप्त करते हैं। इस क्रिया में बने Cao को जल में घोलकर बुझा हुआ चूना प्राप्त कर लेते हैं।
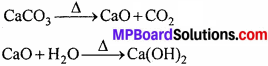
अमोनिया पुनः प्राप्ति स्तंभ में NH4Cl तथा Ca(OH)2 को गर्म करके NH3 प्राप्त करते हैं।
![]()
प्रश्न 13.
पोटैशियम कार्बोनेट सॉल्वे विधि द्वारा नहीं बनाया जा सकता है, क्यों?
उत्तर:
पोटैशियम कार्बोनेट को सॉल्वे विधि से नहीं बनाया जा सकता, क्योंकि पोटैशियम कार्बोनेट जल में अत्यधिक विलेय होने के कारण अवक्षेपित नहीं होता है।
प्रश्न 14.
Li2CO3 कम ताप पर एवं Na2CO3 उच्च ताप पर क्यों विघटित होता है ?
उत्तर:
Li+ आयन आकार में छोटे होते हैं, जो छोटे ऋणायन ऑक्साइड O2-के साथ, CO32- की तुलना में स्थायी जालक बनाते हैं इसलिए Li2CO3 निम्न ताप पर Li2O में विघटित होता है। जबकि Na+ आयन बड़े आकार का होता है, जो बड़े ऋणायन CO32- के साथ O2-आयन की तुलना में स्थायी जालक बनाता है। अतः Na2CO3 अत्यधिक स्थायी है, जो उच्च ताप पर ही Na2O में विघटित होता है।
प्रश्न 15.
क्षार धातुओं के निम्नलिखित यौगिकों की तुलना क्षारीय मृदा धातुओं के संगत यौगिकों से विलेयता एवं तापीय स्थायित्व के आधार पर कीजिए –
(a) नाइट्रेट
(b) कार्बोनेट
(c) सल्फेट।
उत्तर:
(a) क्षार तथा क्षारीय मृदा धातुओं में नाइट्रेट –
1. क्षार तथा क्षारीय मृदा धातुओं के नाइट्रेट जल में अति विलेय होते हैं।
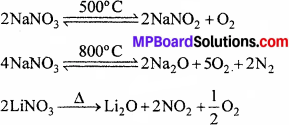
2. क्षार धातुओं के नाइट्रेट (लीथियम नाइट्रेट के अतिरिक्त) उच्च ताप पर नाइट्राइड में अपघटित हो जाते हैं। अधिक ताप पर पुनः गर्म करने पर, ऑक्साइड प्राप्त होते हैं। क्षारीय मृदा धातुओं के नाइट्रेट (बेरियम नाइट्रेट के अतिरिक्त) गर्म करने पर संगत ऑक्साइड देते हैं तथा NO2 तथा O2 का मिश्रण मुक्त होता है।
![]()
(b) क्षार तथा क्षारीय मृदा धातुओं में कार्बोनेट –
1. क्षार धातुओं के कार्बोनेट 1273 K तक स्थायी होता है। इससे अधिक ताप पर ये पिघलकर संगत ऑक्साइड बनाते हैं।
![]()
अपेक्षाकृत कम स्थायी है तथा शीघ्रता से अपघटित हो जाती है।
![]()
सभी क्षारीय मृदा धातुओं के कार्बोनेट गर्म करने पर संगत धातु ऑक्साइड तथा CO2 देते हैं।
![]()
क्षारीय मृदा धातुओं के कार्बोनेटों का तापीय स्थायित्व वर्ग में नीचे जाने पर क्रमशः घटता है। BeCO2 सबसे कम स्थायी है।
2. सभी क्षार धातु कार्बोनेट सामान्यतः जल में विलेय होते हैं तथा इनकी विलेयता वर्ग में नीचे जाने पर क्रमशः बढ़ती है, क्योंकि इनकी जलयोजन ऊर्जा की अपेक्षा जालक ऊर्जा अधिक शीघ्रता से घटती है। क्षारीय मृदा धातुएँ कार्बोनेट जल में विलेय होती है तथा इनकी विलेयता वर्ग में नीचे जाने पर क्रमशः घटती है। यद्यपि ये CO2 की उपस्थिति में अधिक विलेय होती है।
(c) क्षार तथा क्षारीय मृदा धातुओं में सल्फेट –
1. क्षार धातुओं के सल्फेट (Li2SO4 के अतिरिक्त) ताप स्थायी होते हैं, जबकि क्षारीय मृदा धातुओं के सल्फेट गर्म करने पर अपघटित हो जाते हैं। इनका ताप स्थायित्व वर्ग में नीचे की ओर जाने पर क्रमशः बढ़ता है।
2. क्षार धातु सल्फेट (Li2SO4 के अतिरिक्त) जल में विलेय होते हैं। क्षारीय मृदा धातुओं के सल्फेटों की विलेयता वर्ग में नीचे की ओर जाने पर क्रमशः घटती है। BeSO4 तथा MgSO4 जल में शीघ्र विलेय हैं, जबकि BaSO4 जल में पूर्णतया अविलेय है।
प्रश्न 16.
सोडियम क्लोराइड से प्रारम्भ करके निम्नलिखित को आप किस प्रकार बनाएँगे –
- सोडियम धातु
- सोडियम हाइड्रॉक्साइड
- सोडियम परॉक्साइड
- सोडियम कार्बोनेट।
उत्तर:
1. सोडियम धातु:
इसे NaCl (40%) तथा CaCl2(60%) के गलित मिश्रण द्वारा 273K पर डाउन्स सेल में वैद्युत-अपघटन द्वारा बनाया जाता है। कैथोड पर मुक्त Na को केरोसीन (मिट्टी के तेल)में एकत्रित करते हैं, जबकि Cl2 एनोड पर मुक्त होती है।
![]()
कैथोड पर – Na+ + e →Na(s)
एनोड पर – 2Cl– → Cl2(g) + 2e–
2. सोडियम हाइड्रॉक्साइड:
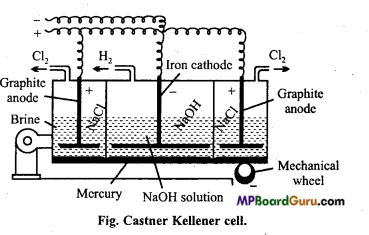
इसे कास्टनर – कैलनर सेल में NaCl के जलीय संतृप्त विलयन (ब्राइन) के वैद्युत – अपघटन द्वारा प्राप्त करते हैं। इस सेल में मर्करी कैथोड तथा कार्बन एनोड का प्रयोग करते हैं। सोडियम धातु जो कैथोड पर मुक्त होती है, मर्करी के साथ संयोग करके सोडियम अमलगम बनाती है। Cl2 गैस एनोड पर मुक्त होती है।
![]()
एनोड पर – 2Cl– → Cl2(g) + 2e–
इस प्रकार प्राप्त सोडियम अमलगम की क्रिया जल से कराने पर सोडियम हाइड्रॉक्साइड तथा हाइड्रोजन गैस मुक्त होती है।
2Na – अमलगम + 2H2O → 2NaOH + 2Hg + H2
3. सोडियम परॉक्साइड:
गलित NaCl के वैद्युत – अपघटन से प्राप्त गलित सोडियम धातु को वायु की अधिकता में गर्म करने पर सोडियम ऑक्साइड प्राप्त होता है। इससे वायु की पुनः क्रिया कराने पर Na2O2 प्राप्त होता है।
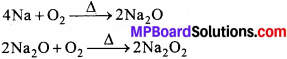
4. सोडियम कार्बोनेट- इसे सॉल्वे अमोनिया प्रक्रम द्वारा बनाया जाता है। अमोनियाकृत सान्द्र ब्राइन विलयन (NaCl के जलीय विलयन) में CO2 प्रवाहित करने पर, सोडियम हाइड्रोजन कार्बोनेट अवक्षेपित होता है। जिसे पुनः गर्म कराने पर सोडियम कार्बोनेट प्राप्त होता है।
![]()
प्रश्न 17.
क्या होता है, जब –
- मैग्नीशियम को हवा में जलाया जाता है
- बिना बुझे चूने को सिलिका के साथ गर्म किया जाता है
- क्लोरीन को बुझे चूने से क्रिया कराया जाता है
- कैल्सियम नाइट्रेट को गर्म किया जाता है।
उत्तर:
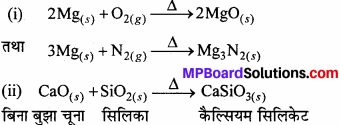
(iii) यह Cl2 से क्रिया करके कैल्सियम हाइपोक्लोराइड बनाता है।
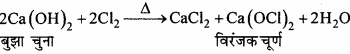
![]()
प्रश्न 18.
निम्नलिखित में से प्रत्येक के दो-दो उपयोग बताइए –
(a) कास्टिक सोडा
(b) सोडियम कार्बोनेट
(c) बिना बुझा चूना।
उत्तर:
(a) कास्टिक सोडा –
- इसका उपयोग साबुन, कागज, कृत्रिम रेशम आदि बनाने में होता है।
- वस्त्र उद्योग में सूती कपड़ों के मर्सरीकरण में इसका प्रयोग होता है।
(b) सोडियम कार्बोनेट –
- इसका प्रयोग जल के मृदुकरण, धुलाई तथा निर्मलन में करते हैं।
- इसका उपयोग काँच, साबुन, बोरेक्स कास्टिक सोडा के निर्माण में होता है।
(c) बिना बुझा चूना –
- इसका प्रयोग कास्टिक सोडा से धावन सोडा बनाने में करते हैं।
- इसका प्रयोग शर्करा के शुद्धिकरण में तथा रंजकों के निर्माण में करते हैं।
प्रश्न 19.
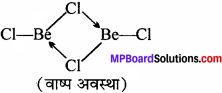
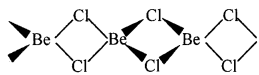
निम्नलिखित की संरचना बताइए –
- BeCl(वाष्य)
- BeCl2(ठोस)।
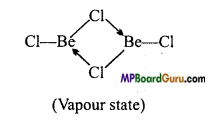
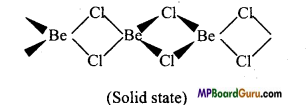
उत्तर:
1. वाष्प अवस्था में यह सेतु बंधित क्लोराइड द्विलक की भाँति रहता है।
2. ठोस अवस्था में, यह क्लोरीन आबंध के साथ बहुलक श्रृंखला संरचना प्रदर्शित करता है।
प्रश्न 20.
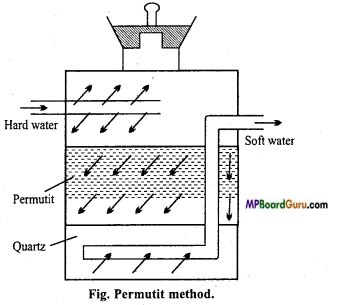
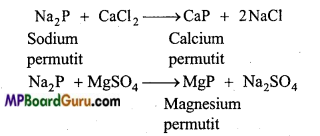
सोडियम एवं पोटैशियम के हाइड्रॉक्साइड एवं कार्बोनेट जल मे विलेय है, जबकि मैग्नीशियम एवं कैल्सियम के संगत लवण जल में अल्प विलेय है। समझाइए।
उत्तर:
![]()
∆Hविलयन का मान जितना अधिक ऋणात्मक होता है, यौगिक की विलेयता उतनी ही कम होती है। सोडियम तथा पोटैशियम हाइड्रॉक्साइड तथा कार्बोनेटों की जल योजन ऊर्जा उनकी जालक ऊर्जा से अधिक होती है। अतः ये जल में विलेय होते हैं। मैग्नीशियम तथा कैल्सियम हाइड्रॉक्साइड की जालक ऊर्जा इनकी जलयोजन ऊर्जा से अधिक होती है। अतः ये जल में अल्प विलेय होते हैं।
प्रश्न 21.
निम्नलिखित की महत्व बताइए –
(a) चूना पत्थर
(b) सीमेंट
(c) प्लास्टर ऑफ पेरिस।
उत्तर:
(a) चूना पत्थर (CaCO3):
- इसे मैग्नीशियम कार्बोनेट के साथ आयरन जैसी धातुओं के निष्कर्षण में गालक के रूप में प्रयोग करते हैं।
- इसका प्रयोग ऐन्टासिड, टूथपेस्ट में अपघर्षक के रूप में, च्यूइंगम में संघटक तथा सौन्दर्य प्रसाधनों के रूप में भी करते हैं।
(b) सीमेंट:
- यह भवन निर्माण हेतु एक महत्वपूर्ण यौगिक है।
- इसका उपयोग काँक्रीट, प्रगलित काँक्रीट, प्लास्टीरंग, पुल-निर्माण, भवन-निर्माण आदि में किया जाता है।
(c) प्लास्टर ऑफ पेरिस:
- इसका उपयोग भवन निर्माण तथा टूटी हुई हड्डियों के प्लास्टर में होता है।
- इसका उपयोग दंत चिकित्सा, अलंकरण कार्य तथा मूर्तियों एवं अर्द्धप्रतिमाओं को बनाने में भी होता है।
प्रश्न 22.
लीथियम के लवण साधारणतया जलयोजित होते हैं, जबकि अन्य क्षार धातुओं के लवण साधारणतया निर्जलीय होते है, क्यों?
उत्तर:
क्षार धातुओं में सबसे छोटा आकार होने के कारण Li+ की जलयोजन कार्य सबसे कम होती है। यही कारण है कि लीथियम लवण सामान्यतः जलयोजित होते हैं तथा अन्य क्षार धातुओं के लवण साधारणतः निर्जलीय होते हैं।
![]()
प्रश्न 23.
LiF जल में लगभग विलेय होता है, जबकि LiCl न सिर्फ जल में, बल्कि एसीटोन में भी विलेय होता है। कारण बताइए, अन्य क्षार धातुओं के लवण साधारणतया निर्जलीय होते है। क्यों ?
उत्तर:
LiF, उच्च जालक ऊर्जा के कारण जल में लगभग विलेय होता है। परन्तु LiCl जल में विलेय होता है। LiCl अपनी विशिष्ट सहसंयोजी प्रकृति के कारण, एसीटोन में भी विलेय होता है। (चूँकि सहसंयोजी प्रकृति, ऋणायन के आकार के साथ बढ़ती है। अतः विलेयता का क्रम निम्न है –
LiF < LiCl< LiBr < Lil
प्रश्न 24.
जैव द्रवों में सोडियम, पोटैशियम, मैग्नीशियम एवं कैल्सियम की सार्थकता बताइए।
उत्तर:
सोडियम तथा पोटैशियम आयन अंतराकाशी द्रव में महत्वपूर्ण भूमिका निभाते हैं। ये आयन शिरा-संकेतों के संचरण में भाग लेते हैं। पोटैशियम आयन कोशिका द्रव में प्रचुरता में पाए जाने वाले धनायन हैं। जहाँ ये अनेक एन्जाइमों को सक्रिय करते हैं, ग्लूकोस के ऑक्सीकरण से ATP के निर्माण में भाग लेते हैं।
पौधे में, प्रकाश अवशोषण के लिए मुख्य रंजक क्लोरोफिल होता है। क्लोरोफिल में मैग्नीशियम होता है। शरीर में कैल्सियम का 99% भाग हड्डियों तथा दांतों में होता है। यह अंतर तांत्रिकीय पेशीय कार्यप्रणाली, अंतर तांत्रिकीय प्रेषण, कोशिका झिल्ली अखंडता तथा रक्त स्कंदन में महत्वपूर्ण भूमिका निभाता है।
प्रश्न 25.
क्या होता है, जब –
(a) सोडियम धातु को जल में डाला जाता है।
(b) सोडियम धातु को हवा की अधिकता में गर्म किया जाता है।
(c) सोडियम परॉक्साइड को जल में घोला जाता है।
उत्तर:
(a) H2 गैस मुक्त होती है, जो अभिक्रिया में अत्यधिक ऊष्मा उत्पन्न करने के कारण आग पकड़ लेती है।
2Na2(s) + 2H2O(l) → 2NaOH(aq)+ H2(g)
(b) Nazo की समान मात्रा के साथ Na20, प्राप्त होता है।
![]()
(c) HO2 बनता है।
Na2O2(s) + 2H2O(l) → 2NaOH(aq) + H2O2(l).
प्रश्न 26.
निम्नलिखित में से प्रत्येक प्रेक्षण पर टिप्पणी लिखिए –
(a) जलीय विलयनों में क्षार धातु आयनों की गतिशीलता Li+ < Na+ <K+ <Rb+ <Cs+ क्रम में होती है।
(b) लीथियम ऐसी एकमात्र क्षार धातु है, जो नाइट्राइड बनाती है।
(c) M2+(aq) + 2e– → M(s); हेतु E° ( जहाँ, M = Ca, Sr या Ba) लगभग समान है।
उत्तर:
(a) आयन का आकार जितना कम होता है, जलयोजन उतना ही अधिक होता है तथा आयन का जलयोजन जितना अधिक होता है, उसकी आयनिक गतिशीलता उतनी ही कम होती है। अतः जलयोजन क्षमता का क्रम निम्न होगा –
Li+ < Na+ <K+ <Rb+ <Cs+
अतः आयनिक गतिशीलता विपरीत क्रम में बढ़ेगी –
Li+(aq) < Na+(aq) <K+(aq) <Rb+(aq) <Cs+(aq)
(b) अपने छोटे आकार के कारण, क्षार धातुओं में केवल लीथियम नाइट्राइड बनाती है।
(c) M2+ + 2e– M(s); जहाँ, M = Ca, Sr, Ba के लिए E° का मान लगभग समान होता है। किसी भी इलेक्ट्रोड के लिए E का मान निम्नलिखित तीन कारकों पर निर्भर करता है –
- वाष्पन एन्थैल्पी
- आयनन एन्थैल्पी
- जलयोजन एन्थैल्पी
चूँकि इन तीनों कारकों का संयुक्त प्रभाव Ca, Sr तथा Ba के लिए लगभग समान रहता है। अतः इनके इलेक्ट्रोड विभव का मान लगभग स्थिर रहता है।
प्रश्न 27.
निम्नलिखित में से प्रत्येक प्रेक्षण पर टिप्पणी लिखिए –
(a) Na2CO3 का विलयन क्षारीय होता है।
(b) क्षार धातुएँ उनके संगलित क्लोराइडों के वैद्युत-अपघटन से प्राप्त की जाती है।
(c) पोटैशियम की तुलना में सोडियम अधिक उपयोगी है।
उत्तर:
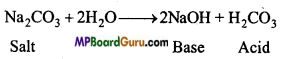
(a) Na2CO3 एक दुर्बल अम्ल (H2CO3) तथा प्रबल क्षार का लवण है। अतः जल-अपघटन कराने पर यह क्षार (NaOH) देता है, जिसके कारण इसका जलीय विलयन क्षारीय होता है।
Na2CO3(s) + 2H2O(l) → 2NaOH(aq) + H2CO3(aq)
(b)1. क्षार धातुएँ प्रबल अपचायक होती है, अतः इन्हें इनके ऑक्साइड तथा यौगिकों से निष्कर्षित नहीं किया जा सकता है।
2. अत्यधिक धनात्मक प्रकृति के कारण इनके लवणों के विलयन से इन्हें किसी अन्य धातु द्वारा विस्थापित नहीं किया जा सकता है।
3. क्षार धातुएँ अपने लवणों के जलीय विलयन के विद्युत्-अपघटन विधि द्वारा प्राप्त नहीं की जा सकती है, क्योंकि कैथोड पर Na धातु की अपेक्षा H, मुक्त होती है। यही कारण है कि क्षार धातुएँ अपने गलित क्लोराइडों के विद्युत्-अपघटन द्वारा प्राप्त होती है।
![]()
वैद्युत अपघटन में निम्नलिखित क्रियाएँ होती हैं –
कैथोड पर – 2cl– → Cl2 + 2e–
एनोड पर – 2Na+ + 2e– →2Na
(c) पोटैशियम की तुलना में सोडियम अधिक महत्वपूर्ण है क्योंकि यह क्रियाशील तो है परन्तु पोटैशियम की भाँति अतिक्रियाशील नहीं है। सोडियम के अन्य उपयोग हैं –
- नाभिकीय संयंत्रों में शीतलक के रूप में।
- पेट्रोल के लिए अपस्फोटन-रोधी पदार्थ, टेट्राएथिल लेड (TEL) के निर्माण में।
4C2H2Cl + 4Na – Pb →(C2H5)4Pb + 3Pb + 4NaCl
प्रश्न 28.
निम्नलिखित के मध्य क्रियाओं के संतुलित समीकरण लिखिए –
- Na2O2 एवं जल
- KO2 एवं जल
- Na2O2 एवं CO2.
उत्तर:
- Na2O2(s) +2H2O(l) → 2NaOH(aq) + H2O2(aq)
- 2KO2(s) + H2O(l) →2KOH(aq) + \(\frac { 3 }{ 2 }\)O2(g)
या 2KO2(s) + H2O(l) →2KOH(aq) + \(\frac { 3 }{ 2 }\)O2(g) - Na2O + CO2 → Na2CO3 .
प्रश्न 29.
आप निम्नलिखित तत्वों को कैसे समझाएँगे –
- BeO जल में अविलेय है, जबकि BeSO4 विलेय है।
- Bao जल में विलेय है, जबकि BaSO4 अविलेय है।
- एथेनॉल में dil. KI की तुलना में अधिक विलेय है।
उत्तर:
1. BeO जल में अविलेय है, क्योंकि BeO की जालक ऊर्जा का मान जलयोजन ऊर्जा से अधिक है, जबकि BeSO जल में घुलनशील है क्योंकि इसकी जलयोजन ऊर्जा जालक ऊर्जा से अधिक है।
2. दूसरे वर्ग के ऑक्साइड की विलेयता के लिए दोनों जालक ऊर्जा और जलयोजन ऊर्जा वर्ग में नीचे जाते समय घटती है, क्योंकि धनायन का आकार बढ़ता है। लेकिन जालक ऊर्जा, जलयोजन की अपेक्षा अधिक तीव्रता से घटती है। अत: BaO जल में घुलनशील है, क्योंकि जलयोजन ऊर्जा जालक ऊर्जा से अधिक है। परन्तु दूसरी ओर BaSO4 जल में अविलेय है। जालक ऊर्जा की प्रबलता समान रहती है, क्योंकि ऋणायन का आकर इतना बड़ा है कि धनायन का आकार बढ़ने से कोई प्रभाव नहीं पड़ता। अत: BaSO4 के लिए जालक ऊर्जा मान जलयोजन ऊर्जा से अधिक है।
3. एथेनॉल में Lil अधिक घुलनशील है, KI की तुलना में Lil में Li4 आयन का आकार छोटा है और घुलनशील है KI आयनिक यौगिक है अतः एथेनॉल में कम घुलनशील है।
प्रश्न 30.
इनमें से किस क्षार धातु का गलनांक न्यूनतम है-
(a) Na,
(b) K,
(c) Rb,
(d) Cs.
उत्तर:
(d) सीज़ियम का गलनांक न्यूनतम है (312K)।
प्रश्न 31.
निम्नलिखित में से कौन-सी क्षार धातु जलयोजित लवण देती है –
(a) Li,
(b) Na,
(c) K,
(d) Cs.
उत्तर:
(a) लीथियम अकेला ऐसा क्षार तत्व है, जो जलयोजित लवण देती है।
प्रश्न 32.
निम्नलिखित में कौन-सी क्षारीय मृदा धातु कार्बोनेट ताप के प्रति सबसे अधिक स्थायी है –
(a) MgCO3
(b) CaCO3
(c) SrCO3
(d) BaCO3
उत्तर:
(d) BaCO3 ताप के प्रति अधिक स्थाई है, यह 1633K पर वियोजित होता है।
s-ब्लॉक तत्त्व अन्य महत्वपूर्ण प्रश्न
s-ब्लॉक तत्त्व वस्तुनिष्ठ प्रश्न
प्रश्न 1.
सही विकल्प चुनकर लिखिए –
प्रश्न 1.
प्लास्टर ऑफ पेरिस होता है –
(a) (CaSO4)2. H2O
(b) CaSO4.2H2O
(c) CaSO4. H2O
(d) CaSO4.
उत्तर:
(a) (CaSO4)2. H2O
प्रश्न 2.
ब्लीचिंग पाउडर का सक्रिय घटक –
(a) Ca(OCl)2
(b) Ca(OCl)Cl
(c) Ca(O2Cl)2
(d) CaCl2O2.
उत्तर:
(b) Ca(OCl)Cl
प्रश्न 3.
द्रव अमोनिया में सोडियम का विलयन निम्न के कारण अपचायक होता है –
(a) Na परमाणु
(b) NaH
(c) NaNH2
(d) e– (NH3)x
उत्तर:
(d) e– (NH3)x
प्रश्न 4.
जल के साथ सोडियम की क्रिया तीव्र होती है, लीथियम की नहीं, क्योंकि लीथियम –
(a) का परमाणु भार अधिक है
(b) एक धातु है
(c) अधिक विद्युत् धनी है
(d) अधिक विद्युत् ऋणी है।
उत्तर:
(d) अधिक विद्युत् ऋणी है।
प्रश्न 5. विभिन्न क्लोराइडों में स्थायित्व का क्रम –
(a) LiCl > KCl > NaCl > CsCl
(b) CsCl > KCI > NaCl > LICl
(c) NaCl > KCl > LiCl > Csci
(d) KCl> CsCl> NaCl> LiCl.
उत्तर:
(a) LiCl > KCl > NaCl > CsCl
प्रश्न 6.
M 2+ की जलयोजन ऊर्जा अधिक होगी इससे –
(a) A3+
(b) Na+
(c) Be2+
(d) Mg3+
उत्तर:
(b) Na+
प्रश्न 7.
क्षारीय मृदा धातुओं का कौन-सा गुण परमाणु-क्रमांक बढ़ने के साथ बढ़ता है –
(a) आयनन ऊर्जा
(b) हाइड्रॉक्साइड की विलेयता
(c) सल्फेट की विलेयता
(d) ऋण-विद्युतता।
उत्तर:
(b) हाइड्रॉक्साइड की विलेयता
प्रश्न 8.
वायु को शुष्क करने के लिये किसका उपयोग ठीक है –
(a) CaCO3
(b) Na2CO3
(c) NaHCO 3
(d) CaO.
उत्तर:
(d) CaO.
प्रश्न 9. किस सल्फेट की विलेयता सबसे कम है –
(a) BaSO4
(b) MgSO4
(c) SrSO4
(d) CaSO4
उत्तर:
(a) BaSO4
प्रश्न 10.
क्षारीय मृदा धातुओं के कार्बोनेटों में तापीय स्थायित्व का क्रम होगा –
(a) BaCO3 > SrCO3 > CaCO3 > MgCO3
(b) BaCO3 > SrCO3 > MgCO3 > CaCO3
(c) CaCO3 > SrCO3 > MgCO3 > BaCO3
(d) MgCO3 > CaCO3 > SrCO3 > BaCO3.
उत्तर:
(d) MgCO3 > CaCO3 > SrCO3 > BaCO3.
प्रश्न 11.
मैग्नीशियम का महत्वपूर्ण अयस्क है –
(a) मेलाकाइट
(b) केसीटेराइट
(c) कार्नेलाइट
(d) गेलेना।
उत्तर:
(c) कार्नेलाइट
प्रश्न 12.
क्षारीय मृदा धातुओं के सल्फेटों की घुलनशीलता समूह में नीचे जाने पर घटती है। इसका कारण है –
(a) गलनांक का बढ़ना
(b) जालक ऊर्जा का बढ़ना
(c) समन्वयन संख्या का बढ़ना
(d) उपर्युक्त सभी।
उत्तर:
(b) जालक ऊर्जा का बढ़ना
प्रश्न 13.
कौन-सा अयस्क लीथियम का नहीं है –
(a) पेंटालाइट
(b) ट्राइफिलाइट
(c) एल्बाइट
(d) स्पोड्यूमीन।
उत्तर:
(c) एल्बाइट
प्रश्न 14.
मैग्नीशियम उपस्थित है इसमें –
(a) हीमोग्लोबीन
(b) क्लोरोफिल
(c) विटामिन B12
(d) विटामिन C.
उत्तर:
(b) क्लोरोफिल
प्रश्न 15.
CaCN2 तथा C के मिश्रण को कहते हैं –
(a) बेराइट
(b) एनहाइड्राइट
(c) नाइट्रोलियम
(d) आइसलैंड स्पॉट।
उत्तर:
(c) नाइट्रोलियम
प्रश्न 2.
रिक्त स्थानों की पूर्ति कीजिए –
- Ca2+ आयन की त्रिज्या K+ आयन से कम है क्योंकि …………. है।।
- Be(OH)2अम्ल तथा क्षार दोनों में विलेय है, क्योंकि इसकी प्रकृति ………….. है।
- लीथियम समूह – 2 के ……………… तत्व से समानता रखता है।
- द्रव अमोनिया में सोडियम धातु नीला रंग देती है यह ………….. के कारण है।
- हाइड्रोलिथ का सूत्र …………… है।
- s – ब्लॉक तत्व प्रबल …………… है।
- क्षारीय मृदा धातु …………. रेडियोधर्मी गुण प्रदर्शित करता है।
- क्षारीय धातु …………….. रेडियोधर्मी गुण प्रदर्शित करता है।
- …………. क्षारीय मृदा धातु हाइड्रॉक्साइड उभयधर्मी है।
- कार्नेलाइट का रासायनिक सूत्र …………… है।
उत्तर:
- धनावेश अधिक
- उभयधर्मी
- Mg
- e– (NH3)x
- CaH2
- अपचायक
- रेडियम
- फ्रान्सियम (Fr)
- Be(OH)2
- KClMgCl2 6H2O.
प्रश्न 3.
उचित संबंध जोड़िए –
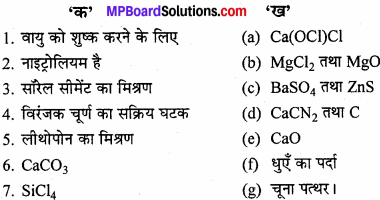
उत्तर:
- (e) CaO
- (d) CaCN2 तथा C
- (b) MgCl2
- (a) Ca(OCI)Cl
- (c) BaSO4
- (g) चूना पत्थर
- (f) धुएँ का पर्दा
प्रश्न 4.
एक शब्द / वाक्य में उत्तर दीजिए –
- क्षारीय मृदा धातुओं को बढ़ती हुई क्रियाशीलता के क्रम में व्यवस्थित कीजिए।
- लीथियम अयस्क के दो नाम सूत्र सहित लिखिए।
- मैग्नीशियम के दो अयस्कों के नाम लिखिए।
- क्षार धातुओं को मिट्टी के तेल में रखा जाता है। क्यों?
- ग्लोबल लवण का आण्विक सूत्र है।
उत्तर:
- क्षारीय मृदा धातुओं की रासायनिक क्रियाशीलता समूह में ऊपर से नीचे आने (Be-Ra) पर बढ़ती है। Be < Mg < Ca < Sr < Ba < Ra
- Li अयस्क के दो नाम व सूत्र –
- स्पोड्यूमीन LiAl(SiO3)2
- लेपिडोलाइट Li2Al2(SiO3)3.F(OH)2
- Mg के दो अयस्कों के नाम –
- कार्नेलाइट
- डोलोमाइट
- अत्यधिक क्रियाशीलता के कारण
- Na2SO4.10H2O
s-ब्लॉक तत्त्व अति लघु उत्तरीय प्रश्न
प्रश्न 1.
एक ऐसे खनिज का नाम तथा सूत्र बताइये जिसमें Ca एवं Mg दोनों उपस्थित होते हैं।
उत्तर:
खनिज का नाम-डोलोमाइट। सूत्र – MgCO3,CaCO3
प्रश्न 2.
फ्लक्स का नाम लिखिए जिसको धात्विक प्रक्रमों में अम्लीय अशुद्धियों को दूर करने के लिये प्रयोग करते हैं।
उत्तर:
- लाइमस्टोन CaCO3
- मैग्नेसाइट MgCO3
प्रश्न 3.
प्लास्टर ऑफ पेरिस का उपयोग प्लास्टर चढ़ाने में किस गुण के कारण किया गया है ?
उत्तर:
प्लास्टर ऑफ पेरिस जल से क्रिया करके सीमेण्ट के समान कठोर हो जाता है। इस गुण के कारण इसका उपयोग टूटी हड्डियों को जोड़ने के लिये प्लास्टर चढ़ाने में किया जाता है।
प्रश्न 4.
IA तथा IIA समूह को s-ब्लॉक तत्व कहते हैं। क्यों?
उत्तर:
IA तथा IIA समूह में उपस्थित सभी तत्वों का इलेक्ट्रॉनिक विन्यास करने पर अंतिम इलेक्ट्रॉन S – उपकोश में प्रवेश करता है, इसलिये इसे s-ब्लॉक तत्व कहते हैं।
उदाहरण –
- Na11 → 1s2 2s22p63s1
- Ca20 → 1s2 2s22p63s23p64s2
प्रश्न 5.
सॉरेल सीमेंट क्या है ? इसका उपयोग किन कार्यों में होता है ?
उत्तर:
MgCl2के सान्द्र विलयन में MgO मिलाया जाये तो MgCl2 2MgO. xH2O संघटन वाले मैग्नीशियम क्लोराइड का एक सफेद पेस्ट बनता है, जो जमकर कड़ा हो जाता है। इसे सॉरेल सीमेन्ट कहा जाता है। इसका उपयोग दाँतों की खोह भरने में तथा चीनी मिट्टी एवं पोर्सलेन के बर्तनों को जोड़ने में किया जाता है।
प्रश्न 6.
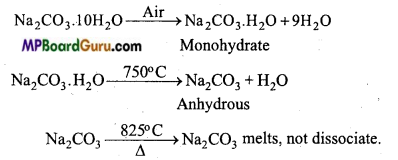
सोडियम कार्बोनेट को वायु में खुला छोड़ देने पर क्या परिवर्तन होता है ? समीकरण सहित समझाइये।
उत्तर:
Na2CO3 10H2O को वायु में खुला छोड़ देने पर इसका क्रिस्टलीय जल धीरे-धीरे निकल जाता है और यह मोनोहाइड्रेट Na2CO2 H2O देता है जो 750°C पर गर्म करने पर निर्जल Na2CO3 देता है जिसे सोडा राख कहते हैं।

प्रश्न 7.
लीथियम हाइड्राइड का प्रयोग अन्य हाइड्राइडों के निर्माण में किया जा सकता है। बेरेलियम हाइड्राइड उनमें से एक है। इसके निर्माण के विभिन्न पद बताइए। इस प्रक्रम में प्रयुक्त रासायनिक समीकरण भी दीजिए।
उत्तर:
BeH2 का निर्माण संगत जटिल क्षारीय धातुओं हाइड्राइडों जैसे लीथियम-ऐल्युमिनियम हाइड्राइड के अपचयन द्वारा करते हैं।
- 8LiH + Al2Cl6 → 2LiAlH4 + 6LiCl
- 2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl2
प्रश्न 8.
कैल्सियम सल्फेट किस प्रकार बनाया जाता है ? इसके उपयोग लिखिए।
उत्तर:
प्रयोगशाला में Ca के ऑक्साइड, कार्बोनेट, क्लोराइड की अभिक्रिया तनु H2SO4 के साथ कराने पर कैल्सियम सल्फेट प्राप्त होता है।
- CaO+ H2SO4 → CaSO4 + H4O
- CaCO3 + H2SO4 → CaSO4 + H2O + CO2
उपयोग:
- चाक बनाने में
- सीमेंट उद्योग में
- प्लास्टर ऑफ पेरिस बनाने में
- खाद के रूप में।
प्रश्न 9.
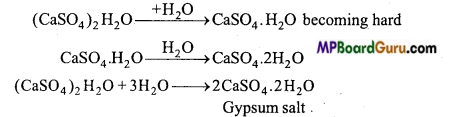
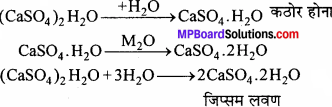
जिप्सम किसे कहते हैं ? इससे प्लास्टर ऑफ पेरिस कैसे बनाते हैं ?
उत्तर:
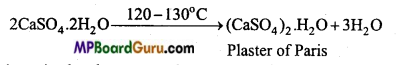
कैल्सियम सल्फेट CaSO4 2H2O को जिप्सम कहते हैं । जिप्सम को 120 – 130°C तक गर्म करने पर इसमें से तीन चौथाई क्रिस्टल जल निकल जाता है तथा प्लास्टर ऑफ पेरिस प्राप्त होता है।
![]()
प्लास्टर ऑफ पेरिस जल अवशोषित करके पुनः जिप्सम में परिवर्तित हो जाता है।
प्रश्न 10.
अनबुझा चूना बनाते समय भट्टी का ताप 1000°C से अधिक गर्म नहीं करते।रासायनिक समीकरण सहित समझाइये।
उत्तर:
अनबुझा चूना बनाते समय भट्टी का ताप 1000°C से अधिक नहीं रखते क्योंकि इससे उच्च ताप पर CaO अशुद्धि के रूप में उपस्थित SiO2 से मिलकर गलनीय सिलिकेट बना लेता है।
![]()
प्रश्न 11.
सोडियम को मिट्टी के तेल में रखा जाता है। क्यों?
उत्तर:
सोडियम अत्यन्त क्रियाशील तथा प्रबल धन विद्युती तत्व है। यह वायुमण्डल में उपस्थित 02, नमी तथा कार्बन डाइ-ऑक्साइड के साथ अभिक्रिया करके ऑक्साइड तथा हाइड्राक्साइड बनाता है। इसलिये सोडियम को मिट्टी के तेल में रखा जाता है जिससे वह वायु के संपर्क में न आ सके।
- 4Na + O2 → 2Na2O
- Na2O+ H2O → 2NaOH
- 2NaOH + CO2 → Na2CO3 + H2O
प्रश्न 12.
चूने के पानी का सूत्र लिखिए। इसमें CO2 के प्रवाह से क्या परिवर्तन होगा?
उत्तर:
बुझे हुये चूने का जल में स्वच्छ विलयन चूने का पानी कहलाता है। इसका सूत्र Ca(OH)2 होगा।
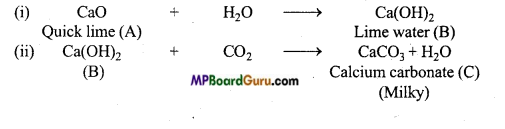
- चूने के पानी में CO2 प्रवाहित करने से CaCO3 बनने के कारण विलयन दूधिया हो जाता है।
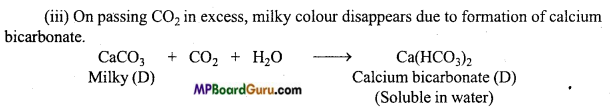
Ca(OH)2 + CO2 → CaCO3 + H2O - चूने के पानी में CO2 को देर तक प्रवाहित करने पर चूने के पानी का दूधियापन समाप्त हो जाता है।
Ca(OH)2 + CO2 + H2O → Ca(HCO3)2
प्रश्न 13.
फोटो रासायनिक सेल में किस धातु का उपयोग होता है और क्यों?
उत्तर:
फोटो रासायनिक सेल में पोटैशियम तथा सीजियम का उपयोग होता है, क्योंकि इनकी आयनन ऊर्जा अत्यन्त कम होती है।
प्रश्न 14.
सोडियम क्लोराइड से सोडियम का निष्कर्षण सामान्य अपचायक से क्यों नहीं किया जा सकता है ?
उत्तर:
सोडियम प्रबल अपचायक है। विद्युत् रासायनिक श्रेणी में इसका स्थान सबसे ऊँचा है। इससे प्रबल अपचायक उपलब्ध न होने से इसे सामान्य अपचायकों द्वारा अपचयित नहीं किया जा सकता है इसे केवल विद्युत् अपघटन द्वारा ही अपचयित किया जाता है।
प्रश्न 15.
Li और Be सहसंयोजी यौगिक बनाने की प्रवृत्ति रखते हैं। समझाइये।
उत्तर:
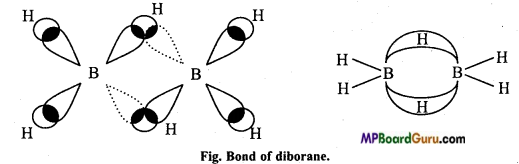
Li और Be परमाणु का आकार बहुत छोटा होता है और उनकी आयनन ऊर्जा अधिक होती है। अतः इनके संयोजकता कोश के इलेक्ट्रॉन नाभिक से दृढ़ता से जुड़े रहते हैं। साथ ही साथ आयनों की ध्रुवण क्षमता उच्च आवेश घनत्व के कारण अधिक होती है। इसलिये Li और Be सहसंयोजी यौगिक बनाने की प्रवृत्ति रखते हैं।
प्रश्न 16.
Li की तुलना में K और Cs का उपयोग फोटो रासायनिक सेल में करते हैं। क्यों?
उत्तर:
Cs तथा K के बड़े आकार के कारण इनकी आयनन ऊर्जा Li की तुलना में अत्यन्त कम है इसलिये सरलता से इलेक्ट्रॉन का त्याग कर सकते हैं। इसलिये फोटो रासायनिक सेल में Li की तुलना में K तथा Cs का उपयोग किया जाता है।
प्रश्न 17.
BeCl2, को वायुमण्डल में रखने पर यह सफेद धूम्र देता है। क्यों?
उत्तर:
BeCl2 आर्द्रता ग्राही होता है। यह वायुमण्डल में उपस्थित नमी को अवशोषित करके जल अपघटित हो जाता है और HCl गैस उत्पन्न करता है। HCl गैस बनने के कारण ही सफेद धूम्र प्राप्त होते हैं।
BeCl2 + 2H2O → Be(OH)2 + 2HCl
प्रश्न 18.
प्रथम वर्ग में ऊपर से नीचे आने पर तत्वों की कठोरता बढ़ती जाती है, क्यों?
उत्तर:
प्रथम वर्ग में ऊपर से नीचे आने पर तत्वों के आकार में वृद्धि के साथ-साथ इनके घनत्वों में भी वृद्धि होती है और उनके परमाणुओं के मध्य आकर्षण बल भी बढ़ता जाता है। जिससे तत्वों की कठोरता बढ़ती जाती है।
s-ब्लॉक तत्त्व लघु उत्तरीय प्रश्न
प्रश्न 1.
s – ब्लॉक तत्व तथा p – ब्लॉक तत्वों को प्रतिनिधि तत्व कहते हैं। क्यों ?
उत्तर:
वे तत्व जिनके बाहरी कोश का इलेक्ट्रॉनिक विन्यास ns1-2 तथा ns2np1-6 होता है, प्रतिनिधि तत्व कहलाते हैं। क्योंकि इस समूह में उपस्थित प्रत्येक तत्व अपने समूह के गुणों का प्रतिनिधित्व करते हैं तथा प्रत्येक समूह के गुण दूसरे समूह के गुण से पूर्णतः भिन्न होते हैं।
(1) s – ब्लॉक तत्व – वे तत्व जिनका अंतिम इलेक्ट्रॉन s – उपकोश में प्रवेश करता है, s – ब्लॉक तत्व कहलाते हैं। इनका सामान्य इलेक्ट्रॉनिक विन्यास ns1-2 होता है।
(2) p – ब्लॉक तत्व-वे तत्व जिनका इलेक्ट्रॉनिक विन्यास करने पर अंतिम इलेक्ट्रॉन p – उपकोश में प्रवेश करता है, p – ब्लॉक तत्व कहलाते हैं। इनका सामान्य इलेक्ट्रॉनिक विन्यास ns2np1-6 होता है।
प्रश्न 2.
क्षार धातु प्रबल अपचायक होते हैं, क्यों?
उत्तर:
क्षार धातुओं के बड़े आकार के कारण इनकी आयनन ऊर्जा कम होती है। जिसके कारण ये संयोजी कोश के इलेक्ट्रॉन को आसानी से त्याग कर ऑक्सीकृत हो जाते हैं तथा इनके मानक इलेक्ट्रोड विभव का मान अधिक ऋणात्मक होता है। इसलिये ये इलेक्ट्रॉन का त्याग कर सरलता से M+ आयन बनाते हैं। इसलिये ये प्रबल अपचायक की तरह कार्य करते हैं।
प्रश्न 3.
BeSO4 तथा MgSO4 जल में शीघ्र विलेय है जबकि CaSO4 SrSO4 तथा BaSO4 अविलेय है। क्यों?
उत्तर:
क्षारीय मृदा धातुओं की जालक ऊर्जा, सल्फेट आयन के वृहद् आकार के कारण लगभग समान होती है। अतः इनकी विलेयता जलयोजन ऊर्जा पर निर्भर करती है, जो वर्ग में नीचे जाने पर क्रमशः घटती है। Be2+ तथा Mg2+ आयनों की उच्च जलयोजन एन्थैल्पी, जालक एन्थैल्पी कारक को हीन कर देती है जिसके कारण इनके सल्फेट जल में विलेय होते हैं। दूसरी ओर Ca2+, Sr2+ तथा Ba2+ आयनों के लिए जलयोजन एन्थैल्पी कम होती है। अत: यह जालक एन्थैल्पी कारक को हीन नहीं कर पाती है। अतः इनके सल्फेट जल में अविलेय होते हैं।
प्रश्न 4.
जलीय विलयन में लीथियम की अपचायक क्षमता अधिक क्यों होती है ?
उत्तर:
किसी तत्व के जलीय विलयन में इलेक्ट्रॉनों की त्यागने की क्षमता का मापन इलेक्ट्रोड विभव द्वारा करते हैं। यह मुख्यतः निम्नलिखित तीन कारकों पर निर्भर करता है –

अपने आयनों के छोटे आकार के कारण, लीथियम की जलयोजन एन्थैल्पी सर्वाधिक होती है। यद्यपि Li की आयनन एन्थैल्पी, क्षार धातुओं से सर्वाधिक होती है परन्तु जलयोजन एन्थैल्पी भी आयनन एन्थैल्पी से अधिक होती है। अतः उच्च जलयोजन एन्थैल्पी के कारण, लीथियम जलीय विलयन में प्रबलतम अपचायक होता है।
प्रश्न 5.
प्रकाश वैद्युत सेल में लीथियम के स्थान पर पोटैशियम एवं सीजियम क्यों प्रयुक्त किए जाते हैं ?
उत्तर:
पोटैशियम तथा सीजियम की आयनन एन्थैल्पी लीथियम की अपेक्षा अधिक कम होती है। अतः ये धातुएँ प्रकाश में रखने पर इलेक्ट्रॉन आसानी से उत्सर्जित करती है, परन्तु लीथियम ऐसा नहीं कर पाती है। यही कारण है Li की अपेक्षा K तथा Cs का प्रयोग प्रकाश वैद्युत सेल में किया जाता है।
प्रश्न 6.
क्या कारण है कि क्षार धातुएँ M+ प्रकार का धनायन बनाती है, M+2 प्रकार का धनायन नहीं?
उत्तर:
क्षार धातुओं के संयोजी कोश में 1 इलेक्ट्रॉन होता है। इनके बड़े आकार के कारण इनकी आयनन ऊर्जा अत्यन्त कम होती है। इसलिये ये सरलता से इलेक्ट्रॉन का त्याग कर M+ आयन बनाते हैं। इस M+1 आयनिक अवस्था में इनका इलेक्ट्रॉनिक विन्यास अक्रिय गैसों के इलेक्ट्रॉनिक विन्यास के समान अत्यन्त स्थायी होता है। इसलिये इस अवस्था में ये रासायनिक दृष्टि से निष्क्रिय होते हैं और इसकी आयनन ऊर्जा अत्यन्त उच्च होती है। इसी कारण ये M+2 आयन नहीं बनाते।
प्रश्न 7.
क्षार धातुओं में कौन-सी धातु प्रबल अपचायक है तथा क्यों ?
उत्तर:
किसी भी तत्व की अपचायक प्रवृत्ति उसके मानक इलेक्ट्रोड विभव पर निर्भर करती है। वे तत्व जिनका मानक इलेक्ट्रोड विभव ऋणात्मक होता है अपचायक की तरह कार्य करते हैं तथा मानक इलेक्ट्रोड विभव का मान जितना अधिक ऋणात्मक होता है वह तत्व उतना प्रबल अपचायक होता है। Li का मानक इलेक्ट्रोड विभव अत्यधिक उच्च ऋणात्मक मान दर्शाता है। इसलिये यह प्रबल अपचायक है।
प्रश्न 8.
क्षार धातु प्रकृति में मुक्त अवस्था में नहीं प्राप्त होते हैं, क्यों? अथवा, क्षार धातु सदैव आयनिक यौगिक का निर्माण करते हैं, क्यों?
उत्तर:
क्षार धातुओं के बड़े आकार के कारण इनकी आयनन ऊर्जा अत्यन्त कम होती है। इसलिये यह सरलता से इलेक्ट्रॉन का त्याग करके धनायन बना सकते हैं। अर्थात् प्रबल धनविद्युती तथा अत्यधिक क्रियाशील होने के कारण वायुमण्डल में उपस्थित ऋणविद्युती तत्व, जैसे-नमी, CO2 के साथ सरलता से अभिक्रिया करके आयनिक यौगिकों का निर्माण करते हैं। इसलिये प्रकृति में मुक्त अवस्था में प्राप्त नहीं होते हैं।
प्रश्न 9.
क्षार धातु सरलता से ज्वाला परीक्षण देते हैं। क्यों ?
उत्तर:
क्षार धातुओं के बड़े आकार के कारण इनकी आयनन ऊर्जा के मान अत्यन्त कम होते हैं। इसलिये इन क्षारीय धातुओं तथा इनके यौगिकों को जब बुन्सन ज्वाला में गर्म किया जाता है तो संयोजी कोश का इलेक्ट्रॉन उत्तेजित होकर उच्च ऊर्जा स्तर में चला जाता है। कुछ समय पश्चात् यह इलेक्ट्रॉन अतिरिक्त ऊर्जा को दृश्य प्रकाश के रूप में प्रकीर्णित कर अपनी मूल स्थिति में वापस आ जाता है । इस प्रकार प्रकाश के प्रकीर्णन के कारण यह ज्वाला को विशिष्ट रंग प्रदान करते हैं।
प्रश्न 10.
Be तथा Mg ज्वाला परीक्षण नहीं देते हैं। क्यों ?
उत्तर:
Be तथा Mg में s – कक्षक पूर्ण कक्षक के रूप में होता है। इनके छोटे आकार तथा :-कक्षक के पूर्ण कक्षक होने की वजह से इनका स्थायित्व अधिक होता है जिसके कारण इनकी आयनन ऊर्जा उच्च होती है। जिसके कारण इलेक्ट्रॉन को बुन्सन ज्वाला द्वारा उत्तेजित करना संभव नहीं है। दूसरे शब्दों में, दृश्य प्रकाश द्वारा विकिरण संभव नहीं है। इसलिये Mg तथा Be ज्वाला परीक्षण नहीं देते हैं।
प्रश्न 11.
क्षार धातुएँ द्रव अमोनिया में घुलकर नीला विलयन बनाती है, जो प्रबल विद्युत् चालक होते हैं। समीकरण सहित कारण बताइये।
उत्तर:
क्षार धातुओं का द्रव अमोनिया में विलयन अमोनीकृत इलेक्ट्रॉन की उपस्थिति के कारण नीले रंग का होता है। इस विलयन की चालकता अमोनीकृत इलेक्ट्रॉन एवं अमोनिया युक्त धनायन दोनों की उपस्थिति के कारण होती है।
M + (x + y)NH3 → [M(NH3)x]+ + [e(NH3)y]–
प्रश्न 12.
Na क्षारीय है अथवा Na2O, स्पष्ट कीजिये।
उत्तर:
Na2O, Na की तुलना में अधिक क्षारीय है क्योंकि Na2O जल से क्रिया करके NaOH बनाते हैं जबकि Na भी जल से अभिक्रिया करके NaOH बनाता है लेकिन पहले वह Nago बनाता है फिर NaOH
- 4Na + 2H2O → 2Na2O + 2H2
- Na2O + H2O → 2NaOH
प्रश्न 13.
क्षार धातुएँ तथा क्षारीय मृदा धातुओं की तुलना निम्नलिखित बिन्दुओं के आधार परकीजिये –
- N2 के साथ क्रिया
- कार्बोनेट पर ऊष्मा का प्रभाव
- सल्फेटों की जल में विलेयता।
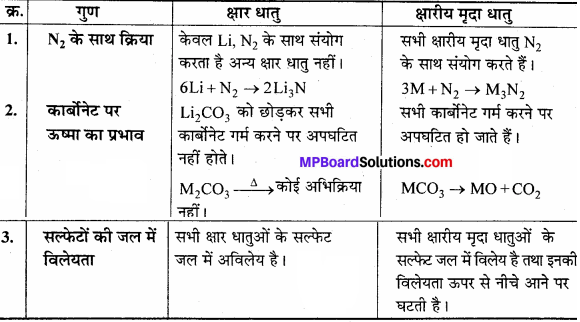
उत्तर:
क्षार धातुएँ तथा क्षारीय मृदा धातु में तुलना –
प्रश्न 14.
क्षार धातुओं को यदि वायुमंडल में खुला रखा जाये तो कुछ समय पश्चात् उनकी धात्विक चमक नष्ट हो जाती है। क्यों?
उत्तर:
प्रत्येक धातु में एक विशिष्ट चमक होती है जिसे धात्विक चमक कहते हैं । क्षार धातुओं के बड़े आकार के कारण इनकी आयनन ऊर्जा अत्यधिक कम होती है। इसलिये यह अत्यधिक क्रियाशील तथा प्रबल धनविद्युती होते हैं तथा वायुमण्डल में उपस्थित नमी, O2 तथा CO2 के साथ अभिक्रिया करके क्षारीय कार्बोनेट बनाते हैं। धातुओं की सतह पर ऑक्साइड तथा कार्बोनेट की पर्त बनने के कारण इनकी धात्विक चमक नष्ट हो जाती है।
प्रश्न 15.
क्षार धातु एवं क्षारीय मृदा धातुओं में प्रमुख अंतर लिखिये।
उत्तर:
क्षार धातु एवं क्षारीय मृदा धातुओं में प्रमुख अंतर –
क्षार धातु:
- ये + 1 संयोजकता दर्शाते हैं।
- इनके हाइड्रॉक्साइड प्रबल क्षार होते हैं।
- इनके कार्बोनेट, सल्फेट, फॉस्फेट जल में विलेय होते हैं।
- इनकी आयनन ऊर्जा का मान अपेक्षाकृत कम होता है।
- ये चमकदार, आघातवर्धनीय एवं तन्य होते हैं।
क्षारीय मृदा धातु:
- ये + 2 संयोजकता दर्शाते हैं।
- इनके हाइड्रॉक्साइड दुर्बल क्षार होते हैं, क्षार धातु की तुलना में।
- इनके यौगिक जल में अविलेय होते हैं।
- इनकी आयनन ऊर्जा का मान उच्च होता है।
- इनके ये गुण क्षार धातु की तुलना में अपेक्षाकृत कम होते हैं।
प्रश्न 16.
LiCl और RbCl में कौन प्रबल आयनिक होगा और क्यों ?
उत्तर:
LiCl की तुलना में RbCl प्रबल आयनिक यौगिक है। क्योंकि Li के छोटे आकार के कारण इसकी आयनन ऊर्जा अत्यधिक उच्च होती है। इसलिये यह सहसंयोजी यौगिक बनाता है जबकि Rb के बड़े आकार के कारण इसकी आयनन ऊर्जा अत्यन्त कम होती है। इसलिये यह सरलता से इलेक्ट्रॉन का त्याग करके धनायन बनाता है। इसलिये इसके यौगिक प्रबल आयनिक प्रवृत्ति दर्शाते हैं।
प्रश्न 17.
क्षार धातु और क्षारीय मृदा धातु में किसके कार्बोनेट जल में विलेय हैं और क्यों?
अथवा
क्षार धातु के कार्बोनेट जल में विलेय हैं जबकि क्षारीय मृदा धातु के कार्बोनेट जल में अविलेय हैं। क्यों?
उत्तर:
क्षार धातु के कार्बोनेट जल में विलेय है क्योंकि इनकी जलयोजन ऊर्जा, जालक ऊर्जा से अधिक होती है जबकि क्षारीय मृदा धातु के छोटे आकार तथा उच्च आवेश घनत्व के कारण इनकी जालक ऊर्जा उच्च तथा जलयोजन ऊर्जा से अधिक होती है। इसलिये क्षारीय मृदा धातु के कार्बोनेट जल में अविलेय है।
प्रश्न 18.
क्षारीय मृदा धातुओं के द्वितीय आयनन विभव का मान प्रथम आयनन विभव से अधिक है फिर भी क्षारीय मृदा धातु + 2 ऑक्सीकरण संख्या दर्शाते हैं + 1 नहीं, क्यों?
उत्तर:
क्षारीय मृदा धातु + 2 आयन का निर्माण करते हैं + 1 आयन का नहीं, क्योंकि
- इनके संयोजी कोश में 2 इलेक्ट्रॉन होते हैं ये तत्व स्थायी इलेक्ट्रॉनिक विन्यास प्राप्त करने के लिये 2e का त्याग कर M+2 आयन का निर्माण करते हैं।
- इस द्विसंयोजी आयन के निर्माण के दौरान जालक ऊर्जा मुक्त होने लग जाती है जो द्वितीय आयनन ऊर्जा के मान को कम कर देती है। इसलिये ये सरलता से द्विसंयोजी आयन का निर्माण करते हैं।
प्रश्न 19.
BeCl2 तथा अन्य क्षारीय मृदा धातुओं के क्लोराइडों में असमानता बताइए।
उत्तर:
- BeCl2 सहसंयोजी यौगिक है जबकि अन्य क्षारीय मृदा धातुओं के क्लोराइड आयनिक है।
- BeCl2 जल में अविलेय है जबकि अन्य क्षारीय मृदा धातुओं के क्लोराइड जल में विलेय है।
- BeCl2 कार्बनिक विलायकों में विलेय है जबकि अन्य क्षारीय मृदा धातुओं के क्लोराइड कार्बनिक विलायकों में विलेय है।
- BeCl2 के गलनांक, क्वथनांक निम्न हैं जबकि अन्य क्षारीय मृदा धातुओं के क्लोराइड के गलनांक, क्वथनांक उच्च हैं।
प्रश्न 20.
BaSO4 की विलेयता CaSO4 से कम है। क्यों?
उत्तर:
क्षारीय मृदा धातुओं के सल्फेटों की विलेयता समूह में ऊपर से नीचे आने पर कम होती है। क्योंकि जालक ऊर्जा तो लगभग समान रहती है। परन्तु समूह में ऊपर से नीचे आने पर परमाणविक त्रिज्या में वृद्धि के कारण जलयोजन ऊर्जा में कमी आती है। जलयोजन ऊर्जा का मान जालक ऊर्जा से कम होने लगता है जिसके कारण विलेयता में कमी आती है।
प्रश्न 21.
Be की आयनन ऊर्जा B से अधिक है। क्यों?
उत्तर:
Be में s – कक्षक पूर्ण कक्षक है। जबकि B में p कक्षक अपूर्ण कक्षक है। अर्धपूर्ण तथा पूर्ण कक्षक अपूर्ण कक्षक की तुलना में अधिक स्थायी होते हैं तथा इनमें से इलेक्ट्रॉन को निकालने के लिये अत्यधिक ऊर्जा की आवश्यकता होती है। इसलिये Be की आयनन ऊर्जा बोरॉन से अधिक है।
प्रश्न 22.
सोडियम कार्बोनेट को अमोनिया सोडा विधि से कैसे बनाया जाता है ? इसके सिद्धान्त को लिखिये।
उत्तर:
इस विधि में पहले NaCl के सान्द्र विलयन को NH3 द्वारा संतृप्त करते हैं । जिससे अमोनियामय सोडियम क्लोराइड बनाता है।
इस अमोनियामय सोडियम क्लोराइड विलयन में CO2 गैस प्रवाहित करते हैं। जिससे अमोनियम बाइकार्बोनेट बनता है जो सोडियम क्लोराइड से क्रिया करके सोडियम बाइ-कार्बोनेट बनाता है।
- NH3 + CO2 + H2O → NH4HCO3
- NH4HCO3 + NaCl → NaHCO3 + NH4Cl
सोडियम बाइ-कार्बोनेट अल्प विलेय होने से अवक्षेप के रूप में नीचे बैठ जाता है। इसे छानकर निस्तापित करने पर सोडियम कार्बोनेट बना लेता है।
2NaHCO3 → Na2CO3 + H2O + CO2
प्रश्न 23.
सोडियम कार्बोनेट से –
- सोडियम बाइ-कार्बोनेट
- सोडियम हाइड्रॉक्साइड
- सोडियम सिलीकेट कैसे प्राप्त करते हैं ?
उत्तर:
- सोडियम कार्बोनेट के जलीय विलयन में CO2 गैस प्रवाहित करने पर सोडियम बाइकार्बोनेट का सफेद अवक्षेप प्राप्त होता है।
NaCO3 + H2O + CO2 → 2NaHCO3 - सोडियम कार्बोनेट को चूने के पानी के साथ उबालने पर सोडियम हाइड्रॉक्साइड बनता है।
Na4CO3 + Ca(OH)2 → 2NaOH + CaCO3 - सोडियम कार्बोनेट को सिलिका के साथ गर्म करने पर सोडियम सिलिकेट बनता है।
Na2CO3 + SiO2 → Na2SiO3 + CO2
प्रश्न 24.
बेकिंग सोडा क्या है ? इसे बनाने की विधि, गुण तथा उपयोग लिखिए।
उत्तर:
परिभाषा-सोडियम हाइड्रोजन कार्बोनेट को बेकिंग सोडा कहते हैं तथा इसका सूत्र NaHCO, है।
बनाने की विधि –
- अमोनिया सोडा विधि में सोडियम बाइ-कार्बोनेट माध्यमिक यौगिक के रूप में मिलता है।
NH4HCO3 + NaCl + NaHCO3 + NH4Cl - सोडियम कार्बोनेट विलयन में CO2 गैस प्रवाहित करने पर सोडियम बाइकार्बोनेट बनता है।
NaCO3 + H2O + CO2 → 2NaHCO3
गुण:
सफेद क्रिस्टलीय पदार्थ जल में अल्प विलेय, जलीय विलयन दुर्बल क्षारीय।
उपयोग:
- बेकिंग पाउडर बनाने में
- पेट की अम्लीयता कम करने की दवा में
- आग बुझाने के यंत्र में।
प्रश्न 25.
सोडियम कार्बोनेट बनाने की ली-ब्लॉक विधि का संक्षिप्त विवरण देते हुये समझाइये कि ली ब्लॉक-विधि से सॉल्वे विधि अच्छी क्यों है ?
उत्तर:
ली-ब्लॉक प्रक्रम – यह प्रक्रम तीन पदों में पूर्ण होता है।
(1) नमक को सान्द्र H2SO4 के साथ गर्म करने पर सोडियम सल्फेट (साल्ट केक) बनता है।
![]()
(2) पिसे हुये साल्ट केक, चूने पत्थर और कोक के मिश्रण को गर्म करने पर सोडियम कार्बोनेट के साथ कैल्सियम सल्फाइड बनता है। CaCO3,Na2CO3 और Cas के इस मिश्रण को काली राख कहा जाता है।
![]()
(3) बारीक पिसी काली राख को जल के साथ उबालने पर Na2CO3 विलेय हो जाता है। अविलेय Cas और CaCO3 के छानकर अलग कर देते हैं। छनित को वाष्पित करके ठोस Na2CO3प्राप्त कर लेते हैं।
सॉल्वे विधि की ली-ब्लॉक से श्रेष्ठता –
- सॉल्वे विधि सस्ती है।
- सॉल्वे विधि में शुद्ध Na2CO3 बनता है।
- सॉल्वे विधि में कोई हानिकारक धूम नहीं निकलते।
- सॉल्वे विधि में बीच में NaHCO3 भी बनता है जो एक उपयोगी यौगिक है।
प्रश्न 26.
सोडियम कार्बोनेट की हारग्रीव-बर्ड सेल विधि का वर्णन कीजिये।
उत्तर:
हारग्रीव बर्ड सेल में कार्बन का एनोड तथा छिद्रयुक्त कॉपर का कैथोड होता है तथा इन्हें ऐस्बेस्टॉस झिल्ली द्वारा पृथक् रखा जाता है। NaCl विलयन का वैद्युत अपघटन कराने पर सोडियम तथा क्लोरीन बनते हैं । सेल में ऐस्बेस्टॉस के बाहरी ओर भाप और CO2भेजी जाती है, जो Na से अभिक्रिया करके Na2CO3 बनाते हैं। इन विलयन का वाष्पन करने पर Na2CO3.10H2O प्राप्त होता है।
- 2NaCl – 2Na+ + 2Cl–
- 2Na+ + 2e– → 2Na
- कैथोड पर – 2Na + 2H2O → 2NaOH + H2.
- 2NaOH + CO2 →Na2CO3 + H2O
![]()
प्रश्न 27.
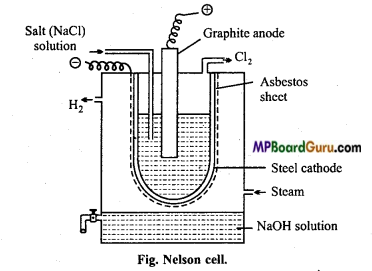
सोडियम हाइड्रॉक्साइड बनाने की नेल्सन सेल विधि का चित्र सहित वर्णन नमक का ग्रेफाइट का ऐनोड कीजिये।
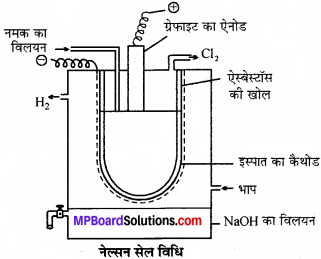
उत्तर:
नेल्सन सेल इस्पात की टंकी में ऐस्बेस्टॉस बेलनाकार ऐस्बेस्टॉस की तह लगी इस्पात की की खोल छिद्रयुक्त नली लगाकर बनाया जाता है। इस्पात की छिद्र युक्त नली कैथोड का कार्य करती है। इस नली में NaCl विलयन भरकर इस्पात की नली में लटका इस्पात का कैथोड देते हैं। कार्बन की छड़ एनोड का कार्य करती है। विद्युत् अपघटन पर सोडियम आयन मुक्त होता है NaOH का विलयन जो ऐस्बेस्टॉस की तह को पार कर कैथोड पर मुक्त होने के बाद टंकी में आने वाली भाप से क्रिया कर NaOH बनाता है।
2NaCl ⇌ 2Na+ + 2Cl–
कैथोड पर – 2Na+ + 2e →2Na
2Na + 2H2O → 2NaOH + H2
![]()
प्रश्न 28.
बुझा हुआ चूना बनाने की विधि, गुण तथा उपयोग लिखिए।
उत्तर:
(1) अनबुझे चूने पर पानी का छिड़काव करने पर बुझा चूना प्राप्त होता है।
CaO + H2O → Ca(OH)2
(2) कैल्सियम लवणों पर क्षार की अभिक्रिया कराने पर Ca(OH)2 प्राप्त होता है।
Ca(NO3)2 + 2NaOH → Ca(OH)2 + 2NaNO3
गुण:
(1) इसे 400°C तक गर्म करने पर कैल्सियम ऑक्साइड बनता है।
![]()
(2) चूने के पानी में CO2 प्रवाहित करने पर CaCO3 का सफेद दूधिया अवक्षेप बनता है।
Ca(OH)2 + CO2 → CaCO3 + H2 O
(3) चूने के पानी में देर तक CO2 प्रवाहित करने पर Ca(HCO3 )2 बनने के कारण दूधियापन समाप्त हो जाता है।
CaCO3 + CO2 + H2O → Ca(HCO3 )2
(4) शुष्क बुझे चूने पर Cl2 गैस प्रवाहित करने पर ब्लीचिंग पाउडर प्राप्त होता है।
Ca(OH)2 + Cl2 → CaoCl2 + H2 O
उपयोग:
- कॉस्टिक सोडा तथा विरंजक चूर्ण के निर्माण में।
- कोल गैस के शोधन में।
- दीवारों पर सफेदी करने में।
- अमोनिया के निर्माण में।
प्रश्न 29.
सोडा लाइम विधि से सोडियम हाइड्रॉक्साइड कैसे बनाते हैं ?
उत्तर:
सोडियम कार्बोनेट के 10 – 20% विलयन को बुझे हुये चूने की उचित मात्रा के साथ भाप के द्वारा 84-85°C ताप गर्म करने पर कैल्सियम कार्बोनेट एवं कास्टिक सोडा बनता है। कास्टिक सोडा विलयन में रहता है जबकि CaCO3 का सफेद अवक्षेप प्राप्त होता है। विलयन को छानकर वाष्पन करने या 98% शुद्ध ठोस कास्टिक सोडा प्राप्त होता है।
Na2 CO3 + Ca[OH]2 → 2NaOH + CaCO3
s-ब्लॉक तत्त्व दीर्घ उत्तरीय प्रश्न
प्रश्न 1.
निम्नलिखित में से प्रत्येक प्रेक्षण पर टिप्पणी लिखिए –
(a) जलीय विलयनों में क्षार धातु आयनों की गतिशीलता Li + > Na+ > K+ > Rb+ > Cs+
(b) लीथियम ऐसी एकमात्र क्षार धातु है, जो नाइट्राइट बनाती है।
(c) M2+(aq) + 2e– →M(s) हेतु E° ( जहाँ, M = Ca, Sr या Ba) लगभग स्थिरांक है।
उत्तर:
(a) आयन का आकार जितना कम होता है, जलयोजन उतना ही अधिक होता है तथा आयन का जलयोजन जितना अधिक होता है, उसकी आयनिक गतिशीलता उतनी ही कम होती है। अतः जलयोजन क्षमता का क्रम निम्न होगा –
Li + > Na+ > K+ > Rb+ > Cs+
अतः आयनिक गतिशीलता विपरीत क्रम में बढ़ेगी
(b) अपने छोटे आकार के कारण, क्षार धातुओं में केवल लीथियम नाइट्राइड बनाती है।
![]()
(c) M2+(aq) + 2e– →M(s)
जहाँ, M = Ca, Sr, Ba के लिए E° का मान लगभग समान होता है। किसी भी M2+/ M इलेक्ट्रोड के लिए E° का मान निम्नलिखित तीन कारकों पर निर्भर करता है –
- वाष्पन एन्थैल्पी
- आयनन एन्थैल्पी
- जलयोजन एन्थैल्पी।
चूँकि इन तीनों कारकों का संयुक्त प्रभाव Ca, Sr तथा Ba के लिए लगभग समान रहता है। अतः इन इलेक्ट्रोड विभव का मान भी लगभग स्थिर रहता है।
प्रश्न 2.
लीथियम अपने समूह के अन्य सदस्यों से अपसामान्य व्यवहार दर्शाता है, क्यों?
उत्तर:
Li अपने समूह के अन्य सदस्यों से अपसामान्य व्यवहार दर्शाता है, क्योंकि –
- इसके परमाणु तथा आयन का आकार छोटा होता है।
- आयनन ऊर्जा उच्च है।
- इलेक्ट्रॉनबंधुता उच्च है।
- d – कक्षक अनुपस्थित है।
अपसामान्य व्यवहार:
- Li अन्य क्षार-धातुओं की तुलना में अधिक कठोर है।
- Li के गलनांक तथा क्वथनांक अन्य क्षार धातुओं की तुलना में उच्च है।
- Liसहसंयोजी यौगिक बनाता है जबकि समूह के अन्य सदस्य आयनिक यौगिक बनाते हैं।
- Li केवल ऑक्साइड बनाता है जबकि अन्य क्षार धातुएँ परॉक्साइड तथा सुपर ऑक्साइड भी बनाते हैं।
- लीथियम हाइड्रॉक्साइड समूह के अन्य धातुओं के हाइड्राइड की तुलना में अधिक स्थायी है।
- लीथियम हाइड्रॉक्साइड दुर्बल क्षार है जबकि अन्य क्षार धातुओं के हाइड्रॉक्साइड प्रबल क्षार है।
- Li नाइट्रोजन के साथ संयोग कर नाइट्राइड बनाता है जबकि समूह की अन्य धातु नाइट्रोजन से संयोग नहीं करती है।
- लीथियम नाइट्रेट गर्म करने पर विघटित होकर Li2O देता है जबकि अन्य क्षार धातु के नाइट्रेट गर्म करने पर अपघटित होकर नाइट्राइट देते हैं।
- 4LiNO3 → 2Li2O + 4NO2 + O2
- 2NaNO3 → 2NaNO2 + O2
- Li2CO3 गर्म करने पर विघटित हो जाता है जबकि अन्य क्षार धातु के कार्बोनेट गर्म करने पर अपघटित नहीं होते हैं।
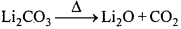
प्रश्न 3.
विकर्ण संबंध क्या है ? Li तथा Mg में विकर्ण संबंध लिखिये।
उत्तर:
विकर्ण संबंध:
द्वितीय आवर्त के कुछ तत्व तृतीय आवर्त के कुछ तत्व के साथ विकर्ण में समानता दर्शाते हैं जिसे विकर्ण संबंध कहते हैं।
Liतथा Mg में विकर्ण संबंध:
- लीथियम की परमाणु त्रिज्या 1.34A तथा Mg की परमाणु त्रिज्या 1.36A लगभग बराबर है।
- Liव Mg+2 की ध्रुवण क्षमता लगभग समान है।
- Li व Mg दोनों कठोर होते हैं।
- Li व Mg की ऋण विद्युतता (1.0 और 1.2) बराबर होती हैं।
- Li तथा Mg दोनों के गलनांक तथा क्वथनांक उच्च हैं।
- Li तथा Mg दोनों N, के साथ अभिक्रिया करके नाइट्राइड बनाते हैं।
- Li तथा Mg दोनों ऑक्सीजन के साथ संयोग करके मोनो ऑक्साइड देते हैं।
- Li तथा Mg दोनों जल को विघटित करके H2 देते हैं।
- Li तथा Mg के कार्बोनेट गर्म करने पर CO2 गैस देते हैं।
- LiOH तथा Mg (OH)2 दोनों दुर्बल क्षार हैं।।
प्रश्न 4.
क्षार धातु तथा क्षारीय मृदा धातु में क्या समानता है ?
उत्तर:
- क्षार धातु तथा क्षारीय मृदा धातु प्रकृति में मुक्त अवस्था में प्राप्त नहीं होते।
- क्षार धातु तथा क्षारीय मृदा धातु के ऑक्साइड जल में विलेय होकर प्रबल क्षार का निर्माण करते हैं।
- क्षार धातु तथा क्षारीय मृदा धातु कोमल तथा चमकीली होती हैं।
- इन्हें वायु में रखने पर इनकी सतह मलिन हो जाती है।
- क्षार धातु तथा क्षारीय मृदा धातु (Be तथा Mg को छोड़कर) ज्वाला परीक्षण देते हैं।
- क्षार धातु तथा क्षारीय मृदा धातु प्रबल अपचायक है।
- क्षार धातु तथा क्षारीय मृदा धातु दोनों आयनिक यौगिक का निर्माण करते हैं।
- दोनों के नाइट्रेट तथा हैलाइड जल में विलेय हैं।
प्रश्न 5.
Be अपने समूह के अन्य सदस्यों की तुलना में अपसामान्य व्यवहार दर्शाता है, क्यों?
उत्तर:
Be अपने समूह के अन्य सदस्यों से अपसामान्य व्यवहार दर्शाता है, क्योंकि
- इसके परमाणु तथा आयन का आकार छोटा होता है।
- आयनन ऊर्जा अत्यधिक उच्च होती है।
- इलेक्ट्रॉनबंधुता उच्च होती है।
- d-कक्षक की अनुपस्थिति।
अपसामान्य व्यवहार:
- Be कठोर है जबकि इस समूह के अन्य सदस्य कोमल धातु होती है।
- अन्य क्षारीय मृदा धातु की तुलना में Be के गलनांक तथा क्वथनांक उच्च होते हैं।
- Be के यौगिक सहसंयोजी होते हैं। जबकि अन्य क्षारीय मृदा धातु के यौगिक आयनिक होते हैं।
- Be अम्लों से सरलता से H2 मुक्त नहीं करता जबकि अन्य क्षारीय मृदा धातु शीघ्रता से H2 मुक्त करती है।
- बेरीलियम कार्बाइड जल अभिक्रिया कराने पर मेथेन देता है, जबकि अन्य सदस्य एसीटिलीन देते हैं ।
Be2C + 2H2O → 2BeO + CH4
CaC2 + 2H20 → Ca(OH)2 + C2H,2 - BeO उभयधर्मी है जबकि अन्य सदस्यों के ऑक्साइड क्षारीय होते हैं।
- Be व Mg को छोड़कर सभी सदस्य ज्वाला परीक्षण देते हैं।
- Be गर्म करने पर भी जल के साथ कोई अभिक्रिया नहीं दर्शाता जबकि अन्य क्षारीय मृदा धातु जल के साथ सरलता से अभिक्रिया दर्शाते हैं।
- Be हाइड्रोजन के साथ मंद गति से अभिक्रिया करता है, जबकि अन्य शीघ्रता से अभिक्रिया करते हैं।
प्रश्न 6.
Be व AI में विकर्ण संबंध लिखिये।
उत्तर:
- Be व AI की परमाणविक त्रिज्या तथा आयनिक त्रिज्या लगभग बराबर है।
- दोनों सहसंयोजी यौगिक बनाते हैं।
- दोनों धातुएँ दुर्बल विद्युती धनी प्रकृति के होते हैं।
- दोनों धातुओं की सान्द्र HNO, से क्रिया कराने पर ये निष्क्रिय होते हैं।
- दोनों शीघ्रता से हाइड्राइड नहीं बनाती।
- Be तथा AI दोनों के ऑक्साइड उभयधर्मी प्रकृति के होते हैं।
- BeO +2HCl→ BeCl2 + H2O
- BeO + 2NaOH → Na2BeO2 + H2O
- Al2O3+6HCl → 2AlCl3 + 3H2O
- Al203 + 2NaOH → 2NaAlO2 + H2O
- दोनों धातुओं के कार्बाइड जल से क्रिया करके मीथेन देते हैं।
Be2C + 2H2O → 2BeO + CH2
Al4C3 + 6H2O → 2AI2O3 + 3CH4 - BeCl2 तथा AlCl3 द्विलक तथा बहुलक के रूप में मिलते हैं।
- Be तथा A1 के ऑक्साइड दुर्बल क्षार हैं।
- BeCl2तथा AlCl3 प्रबल लुईस अम्ल हैं।
प्रश्न 7.
सॉल्वे विधि द्वारा सोडियम कार्बोनेट का निर्माण किस प्रकार किया जाता है ? नामांकित रेखाचित्र खींचिए एवं समीकरण लिखिये।
उत्तर:
जब अमोनियामय NaCl विलयन में CO2 गैस प्रवाहित करते हैं तो अमोनियम बाइकार्बोनेट बनता है। जो NaCl से अभिक्रिया करके सोडियम बाइकार्बोनेट बनाता है।
- NH3 + CO2 + H2O → NH4HCO3
- NH4HCO3 + NaCl → NaHCO3 + NH4Cl
सोडियम बाइकार्बोनेट अल्प विलेय होने से अवक्षेपित होकर नीचे बैठ जाता है। इसे छानकर निस्तापित करने पर Na2co3 प्राप्त होता है।
2NaHCO3 → Na2CO3 + H2O + CO2
उपकरण एवं विधि –
(1) अमोनिया संतृप्त स्तम्भ – इसमें ब्राइन को अमोनिया से संतृप्त करते हैं। अमोनियामय ब्राइन बनता है तथा Ca एवं Mg की अशुद्धि अवक्षेपित होकर नीचे बैठ जाती है।
(2) छन्ना – Ca तथा Mg के अवक्षेप अमोनियामय ब्राइन से पृथक् हो जाते हैं।
(3) शीतकारक-अमोनियामय ब्राइन को ठण्डा करते हैं।
(4) कार्बोनेटीकरण स्तम्भ – अमोनियामय ब्राइन में चूने के भट्टी से प्राप्त CO2 प्रवाहित करने पर सोडियम बाइकार्बोनेट प्राप्त होता है।
- 2NH3 + H2O + CO2 → (NH4)2 CO3
- (NH4)2CO3CO2 + H2O → 2NH4HCO3
- NH4HCO3 + NaCl → NaHCO3 + NaCl
(5) निर्वात् छन्ना – अविलेय NaHCO3 छनकर पृथक् हो जाता है। विलयन में NH4Cl तथा NH4HCO3 शेष रहता है। इसे पुनः प्राप्ति स्तम्भ में भेजा जाता है।
(6) चूने की भट्टी – चूने के पत्थर से CO2 गैस प्राप्त की जाती है।
![]()
CaO जल के साथ क्रिया कर Ca(OH)2 बनाता है जो NH4Cl के साथ अभिक्रिया कर पुन: NH3 देता है।
CaO + H2O + Ca(OH)2
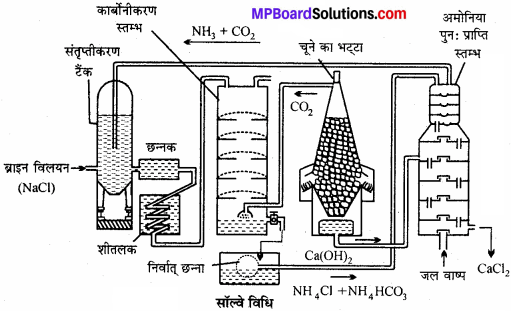
(7) अमोनिया पुनः प्राप्ति स्तम्भ – निर्वात् छन्ने से प्राप्त द्रव पर ऊष्मा तथा बुझे चूने की क्रिया से अमोनिया प्राप्त करते हैं।
- NH4HCO3 → NH3 + H2O+CO
- 2NH4Cl + Ca(OH)2 → CaCl2 + 2NH3 + 2H2O
(8) NaHCO4 का जारण – निर्वात् छन्ना से प्राप्त सोडियम बाइकार्बोनेट प्राप्त होता है। इसे बेलनाकार भट्टियों में गर्म करते हैं जिससे सोडियम कार्बोनेट प्राप्त होता है।
2NaHCO2 → Na2CO3 + H2O + CO2
प्रश्न 8.
जब वर्ग – 1 की एक धातु को द्रव अमोनिया में घोला गया, तो निम्नलिखित प्रेक्षण प्राप्त हुए –
(a) प्रारंभ में नीला विलयन प्राप्त हुआ।
(b) विलयन को सान्द्र करने पर, नीला रंग-काँस्य-रंग में परिवर्तित हो गया। विलयन के नीले रंग की व्याख्या कीजिए। विलयन को कुछ समय तक रखने पर प्राप्त उत्पाद का नाम बताइए।
उत्तर:
(a) वर्ग-1 धातुओं को द्रव अमोनिया में घोलने पर निम्नलिखित अभिक्रिया प्राप्त हुई –
![]()
विलयन का नीला रंग अमोनीकृत इलेक्ट्रॉनों के कारण होता है, जो दृश्य प्रकाश क्षेत्र की संगत् ऊर्जा का अवशोषण करके विलयन को नीला रंग प्रदान करता है।
(b) सान्द्र विलयन में, धातु आयन स्तर बन जाने के कारण नीला रंग, काँस्य रंग में बदल जाता है। नीला विलयन कुछ समय तक पड़े रहने पर हाइड्रोजन को मुक्त करता है तथा ऐमाइड बनते हैं।
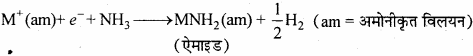
प्रश्न 9.
क्षार धातुओं के परॉक्साइड तथा सुपर ऑक्साइडों का स्थायित्व वर्ग में नीचे की ओर जाने पर घटता है। उदाहरण सहित व्याख्या कीजिए ?
उत्तर:
परॉक्साइड तथा सुपर ऑक्साइडों का स्थायित्व धातु आयन का आकार बढ़ने पर बढ़ता है।
KO2 < RbO2 < CsO2
क्षार धातुओं का ऑक्सीजन के साथ क्रिया करके विभिन्न ऑक्साइड बनाने का कारण, क्षार धातु के धनायन के परितः प्रबल धनात्मक क्षेत्र का निर्माण होता है। Li* का आकार सबसे छोटा है, जिसके कारण यह O2-आयन को पुन: O2 से क्रिया नहीं करने देता है। Na+ का आकार Li से बड़ा है अतः इसका धनात्मक Li+ के क्षेत्र से क्षीण होता है। K+ Rb+ Cs+जैसे बड़े आयन O2-2 आयन को पुन: 02 से क्रिया करके सुपरऑक्साइड (O–2) बनाने देते हैं।
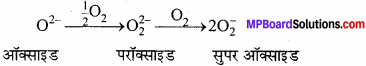
पुनः धातु आयनों का आकार बढ़ने के साथ-साथ परॉक्साइडों तथा सुपरऑक्साइडों के स्थायित्व में भी वृद्धि होती है। इसका प्रमुख कारण जालक ऊर्जा प्रभाव के फलस्वरूप बड़े ऋणायनों का बड़े धनायनों द्वारा स्थायित्व प्रदान करना है।
प्रश्न 10.
सोडियम हाइड्रॉक्साइड प्राप्त करने की कास्टनर केलनर सेल का चित्र सहित वर्णन कीजिए।
उत्तर:
इसमें एक लोहे की आयताकार टंकी होती है जो स्लेट की पट्टियों द्वारा तीन भागों में विभाजित रहती है। ये पट्टियाँ हौज पेंदे को छूती नहीं हैं बल्कि निचला भाग हौज के तले में रखे हुये पारे से ढंका रहता है। पारे की पर्त तीनों भागों को एक-दूसरे से पृथक् रखती है। पारे की पर्त यांत्रिक प्रबन्ध द्वारा इधर – उधर घूमती रहती है।
बाहरी कक्ष में NaCl का विलयन भरा रहता है, जिसमें ग्रेफाइट की छड़ लगी रहती है। ये एनोड का कार्य करती है। बीच ऐनोड वाले भाग में NaOH का तनु विलयन भरा रहता है, जिसमें लोहे की छड़ का बना कैथोड लटका – रहता है। विद्युत् धारा प्रवाहित करने पर पारा प्रेरण बीच का कक्ष एनोड का तथा बाहरी कक्ष कैथोड का कार्य करते हैं।
बाहरी कक्ष में एनोड पर Cl2 मुक्त होती है। सोडियम कैथोड पर मुक्त होकर पारे के साथ सोडियम अमलगम बना लेता है। सोडियम अमलगम उत्क्रेन्द्रीय पट्टियों की सहायता से सेल के मध्य भाग में आता है । यहाँ पर सोडियम अमलगम ऐनोड का कार्य करता है और आयरन की छड़ कैथोड का। इस भाग में NaOH भरा रहता है। विद्युत् धारा प्रवाहित करने पर OH– आयन एनोड पर विसर्जित होते हैं तथा अमलगम में उपस्थित सोडियम से क्रिया कर NaOH बनाते हैं तथा H2 गैस मुक्त करते हैं।
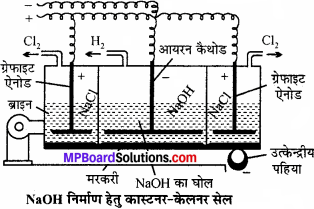
अभिक्रिया-बाहरी कक्ष में –
2NaCl ⇄ 2Na+ + 2Cl–
कैथोड पर –
2Na+ + 2e → 2Na
2Na + xHg → HgxNa2
एनोड पर –
![]()
मध्य कक्ष में –
NaOH ⇄ Na+ + OH–
कैथोड पर –
2NaHg + 2H2O → 2NaOH + 2Hg + H2
Na+ + e → Na
2Na + 2H2O → 2NaOH + H2
एनोड पर –
2OH– → 2OH + 2e
Hgx Na2 + 2OH– → 2NaOH + xHg
प्रश्न 11.
जब कैल्सियम के यौगिक –
(A) में जल मिलाया जाता है तो यौगिक (B) के विलयन का निर्माण होता है। इस विलयन में कार्बन डाइ-ऑक्साइड प्रवाहित करने पर यह यौगिक (C) बनने के कारण दुधिया हो जाता है। कार्बन डाइ-ऑक्साइड की अधिक मात्रा में प्रवाहित करने पर, यौगिक (D) के निर्माण के कारण यह दुधियापन लुप्त हो जाता है तथा यौगिक (A), (B), (C) तथा (D) को पहचानिए तथा बताइए कि अंतिम पद में दुधियापन क्यों समाप्त हो जाता है ?
उत्तर:
यौगिक (B) के विलयन में CO2 प्रवाहित करने पर विलयन का दुधिया होना संकेत करता है कि यौगिक (B) बुझा हुआ चूना [Ca(OH)2] है तथा यौगिक (C) कैल्सियम कार्बोनेट है। चूँकि यौगिक (B), यौगिक (A) में H2O को मिलाने से प्राप्त होता है। अतः यौगिक (A) में बिना बुझा चूना (CaO) है। संगत अभिक्रियाएँ निम्नलखित हैं –
![]()

(iii) CO2 को अधिकता में प्रवाहित करने पर, विलेय कैल्सियम बाइकार्बोनेट बनाने के कारण दुधियापन लुप्त हो जाता है।

प्रश्न 12.
कैल्सियम ऑक्साइड बनाने की विधि, रासायनिक गुण तथा उपयोग लिखिये।
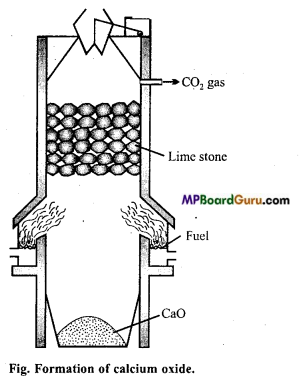
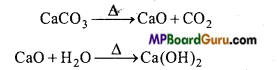
उत्तर:
चूने के पत्थर को गर्म करके कैल्सियम ऑक्साइड बनाते हैं।
![]()
यह अभिक्रिया उत्क्रमणीय है। अतः चूना प्राप्त करने हेतु CO2 को जल्दी-जल्दी-हटाते रहना आवश्यक है। अभिक्रिया का-ताप 900°C होना चाहिये क्योंकि अधिक ताप पर मिट्टी और चूने की अभिक्रिया से गलनीय सिलिकेट बन जाता है। भट्टी में बाजू से दो अँगीठियों में कोयला जलाया जाता है। ऊपर से धीरे-धीरे चूने का पत्थर डालते रहते हैं, जो नीचे आते-आते अपघटित हो जाता है। CO2 गैस ऊपरी भाग से बाहर निकलती है। इसे द्रवित कर सिलेण्डरों में भर लिया जाता है।
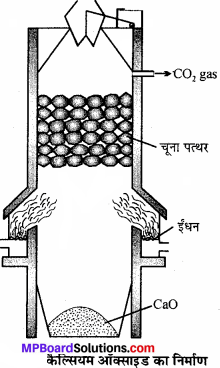
रासायनिक गुण:
(1) नम वायु से नमी तथा CO2 सोखकर Ca(OH)2 तथा CaCO3 बनाता है।
- CaO + H2O Ạ Ca(OH)2
- CaO + CO2 CaCO2
(2) यह एक प्रबल क्षारीय ऑक्साइड है जो अम्लों के साथ अभिक्रिया कर लवण बनाता है।
- CaO + 2HCl → CaCl2 + H2O
- Cao + SiO2→ CaSiO3
(3) अमोनियम लवणों के साथ गर्म करने पर NH, गैस बनती है।
2NH4Cl + CaO → CaCl2 + H2O + 2NH2
(4) कार्बन और कैल्सियम ऑक्साइड को गर्म करने से CaC2 प्राप्त होता है।
![]()
उपयोग:
- धातुकर्म के गालक के रूप में
- ऐल्कोहॉल तथा गैसों को सुखाने में
- लाइम लाइट उत्पन्न करने के लिये
- कोल गैस को शुद्ध करने में
- कागज बनाने में।
प्रश्न 13.
वर्ग-1 के एक तत्व का आयन कोशिकाओं में शिरा-संकेतों के संचरण, शर्करा तथा एमीनो अम्लों के प्रवाह में सहायक है। यह तत्व ज्वाला परीक्षण में ज्वाला के साथ पीला रंग देता है तथा
ऑक्सीजन के साथ ऑक्साइड तथा परॉक्साइड बनाता है।तत्व की पहचान कीजिए तथा इसके परॉक्साइड निर्माण की रासायनिक समीकरणों को लिखिए। यह तत्व ज्वाला के साथ रंग क्यों देता है ?
उत्तर:
ज्वाला परीक्षण में पीले रंग की ज्वाला दर्शाता है कि धातु सोडियम ही होनी चाहिए। यह 0, के साथ क्रिया करके सोडियम परॉक्साइड Na2O2 तथा सोडियम ऑक्साइड, Nao का मिश्रण देता है।

सोडियम की आयनन एन्थैल्पी कम होती है। जब सोडियम धातु या इसके लवण को बुन्सन ज्वाला में गर्म किया जाता है, तब ज्वाला की ऊष्मा बाह्यतम इलेक्ट्रॉन को उच्च ऊर्जा स्तर पर उत्तेजित कर देती है तब ये इलेक्ट्रॉन पुनः अपनी तलस्थ / आद्य अवस्था में आते हैं तो दृश्य क्षेत्र में विकिरण उत्सर्जन के कारण ज्वाला को पीला रंग प्रदान करते हैं।
प्रश्न 14.
निम्नलिखित की महत्ता बताइए –
(a) चूना पत्थर
(b) सीमेंट
(c) प्लास्टर ऑफ पेरिस।
उत्तर:
(a) चूना पत्थर (CaCO3):
- इसे मैग्नीशियम कार्बोनेट के साथ आयरन जैसी धातुओं के निष्कर्षण में गालक के रूप में प्रयोग करते हैं।
- इसका प्रयोग ऐन्टासिड, टूथपेस्ट में अपघर्षक के रूप में, च्यूइंगम में संघटक तथा सौन्दर्य प्रसाधनों में रूपक के रूप में भी करते हैं।
(b) सीमेंट:
- यह भवन निर्माण हेतु एक महत्वपूर्ण यौगिक है।
- इसका उपयोग कांक्रीट, प्रबलित कांक्रीट, प्लास्टरिंग, पुल-निर्माण, भवन-निर्माण आदि में किया जाता है।
(c) प्लास्टर ऑफ पेरिस:
- इसका उपयोग भवन निर्माण तथा टूटी हुई हड्डियों के प्लास्टर में होता है।
- इसका उपयोग दंत-चिकित्सा, अलंकरण कार्य तथा मूर्तियों एवं अर्द्धप्रतिमाओं को बनाने में भी होता है।