Students get through the MP Board Class 11th Chemistry Important Questions Chapter 9 Hydrogen which are most likely to be asked in the exam.
MP Board Class 11th Chapter 9 Hydrogen
Hydrogen Class 11 Important Questions Very Short Answer Type
Question 1.
What are the causes of temporary and permanent hardness of water? Explain.
Answer:
Temporary hardness of water is due to presence of bicarbonates of Ca and Mg. Permanent hardness is due to presence of chlorides and sulphates of calcium and magnesium.
Question 2.
Write chemical reactions to show the amphoteric nature of water.
Answer:
Water is amphoteric in nature. It behaves as an acid and base both. With stronger acids than itself, it acts as a base where as with stronger bases than itself it acts as an acid.
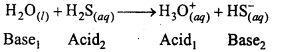
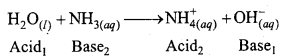
Question 3.
Is demineralized or distilled water useful for drinking purposes? If not, how can it be made useful?
Answer:
Demineralized or distilled water is not useful for drinking purposes. It can be made useful by mixing appropriate amount of some useful salts in it.
Question 4.
Knowing the properties of H2O and D2O, do you think that D2O can be used for drinking purposes?
Answer:
Heavy water, D2O is harmful for living organisms because in heavy water, rate of biochemical reactions decreases.
Question 5.
Beryllium forms covalent hydride whereas calcium forms ionic hydride. Why?
Answer:
Due to high. electronegativity Be (1.5) forms covalent hydride whereas electronegativity of Ca is low.
Question 6.
Explain Uno process of the preparation of hydrogen.
Answer:
Uno’s method : Aluminium is treated with hot caustic soda solution. Reaction proceed briskly. Aluminium dissolve and hydrogen gas is produced.
2 Al + 2NaOH + 2H2O → 2NaAlO2 +3H2 Sodium meta aluminate.
![]()
Question 7.
How is impure hydrogen purified?
Answer:
On passing hydrogen gas on Platinum black or Palladium metal, hydrogen is adsorbed by the metal. This way impure hydrogen is purified because only pure hydrogen can be adsorbed by Palladium.
Question 8.
Write the disadvantages of hard water.
Answer:
Disadvantages of hard water:
- More soap is wasted to produce lather for working clothes.
- Hard water cannot be used in medicine for injections.
- It is harmful for people suffering from arthritis.
Question 9.
Describe Lanes process for the preparation of hydrogen.
Answer:
In this process water vapours are passed over red hot iron at 600- 900° C and hydrogen gas is formed. Reaction is reversible, hence H2 should be periodically removed.
3Fe + 4H2O ⇌ Fe3O4+4H2
Question 10.
Explain the method by which temporary and permanent both types of hardness is removed.
Answer:
Chemical method: In this method calcium hydroxide [Ca(OH)2 ] or sodium carbonate (Na2CO3) or sodium phosphate is mixed in hard water when insoluble salts of Ca and Mg are formed and separated and both temporary and permanent hardness are removed.
Ca(HCO3)2 + Na2CO3 → CaCO3↓ +2NaHCO3
MgCl2+Na2CO3 → MgCO3 ↓ +2NaCl
CaSO4 + Na2CO3 → CaCO3 ↓ +Na2SO4
Question 11.
How is the strength of H2O2 expressed?
Answer:
Strength of Hydrogen peroxide solution: The strength of hydrogen peroxide solution is expressed in terms of the volume of oxygen obtained from it. For example, 10 volume, 20 volume etc. The strength is equal to the volume of oxygen produced at NTP which is obtained by heating one unit of that solution.
The ‘10 volume’ solution of hydrogen peroxide will give 10 ml oxygen at NTP when 1 ml of the sample is heated.
![]()
Question 12.
Write the uses of hydrogen.
Answer:
Uses of hydrogen :
- As a reducing agent,
- For the preparation of ghee by the hydrogenation of oil,
- For the preparation of artificial petrol by reacting with coal powder,
- In glass industry, in cooling the glass slowly.
Question 13.
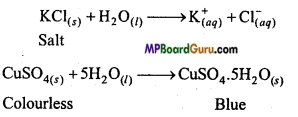
What is the difference in the steps of hydrolysis and hydration?
Answer:
The process of spontaneous reaction of H+ ion and OH– ions of water with the anion and cation of a salt to form the basic acid and water is called hydrolysis.
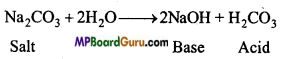
On the other hand, hydration means formation of hydrated ions or hydrated salts by the addition of water in ions and molecules.
Question 14.
What do you expect the nature of hydrides is, if formed by elements of atomic numbers 15, 19, 23 and 44 with dihydrogen ? Compare their behaviour towards water.
Answer:
- Element of Z = 15, Phosphorus, is a member of p-block, thus it a covalent hydride.
- Element of Z = 19, Potassium, is a member of 5-block, thus it is an ionic hydride.
- Element of Z = 23, Vanadium, is a member of rf-block and group-5. It forms interstitial hydride VH16. It is a non-stoichiometric hydride.
- Element of Z = 44, Rutheum, is a member of d-block and group-8. It does not form any hydride because elements of group-7, 8 and 9 do not form hydride (Hydrideless). With water, only ionic hydride, KH reacts to liberate dihydrogen gas.
Question 15.
What is oil gas and how is it obtained?
Answer:
This gas is prepared by the distillated of kerosene oil. A thin layer of kerosene oil is poured in red hot iron retort by which bigger molecules break up into methane, ethylene and acetylene. It is used in burners.
Question 16.
What is coal gas? Write its chemical composition.
Answer:
Coal gas is a mixture of various gases in which hydrogen is also one of the constituent. Percentage composition of coal gas is as follows :
H2 = 43.55%,CH4 = 25.35%,CO2 = 0.3%, CO = 4.11% N2 =2.12%,
O2 = 0-1.5%
Apart from being used as a fuel, coal gas is also used for the production of light.
Question 17.
A gas is produced on reacting sodium with water. Write its name and formula.
Answer:
By the reaction of water on extremely reactive metals like – sodium, potassium, calcium at ordinary temperature, hydrogen gas is released.
2Na + 2H2O → 2NaOH+H2
Ca + 2H2O → Ca(OH)2+H2.
Question 18.
What is the cause of hardness of water and hardness is of how many types?
Answer:
Hardness of water is due to the presence of soluble bicarbonates, sulphates, chlorides, salts of calcium and magnesium.
1. Temporary hardness: When hardness of water is removed by boiling or by simple methods then it is known as temporary hardness. Temporary hardness is due to bicarbonates of Ca and Mg.
2. Permanent hardness: If hardness is not removed by boiling or by simple methods, it is known as permanent hardness. It is due to chlorides and sulphates of Ca and Mg.
![]()
Question 19.
What is hard and soft water?
Answer:
Soft water: Water which easily form good lather with water is called soft water. Distilled water, rain water, river water etc. are soft water.
Hard water: Water which does not form good lather with water easily is called hard water. It gives less lather and soap spreads and form clots, due to this lot of soap is wasted.
Question 20.
What is distilled water? Write its one use.
Answer:
Pure water obtained by distillation method is known as distilled water. It is used for the preparation of medicines and solutions in the laboratory.
Question 21.
What is the utility of heavy water?
Answer:
- Heavy water is mainly used as a moderator in nuclear reactors.
- It is used as a tracer compound in the study of mechanism of reactions.
- It is used for the preparation of other deuterium compounds like CD4, D2SO4etc.
Question 22.
Acidic solution of hydrogen peroxide behaves as an oxidizing as well as reducing agent. Explain it with the help of chemical equations.
Answer:
- H2O2 oxidizes acidified KI to iodine.
2KI + 2H2O2 + H2SO4 → I2 + K2SO4 + 2H2O - In alkaline medium H202 reduces KMnO4 to MnO2.
2KMnO4 + 3H2O2 →2MnO2+2KOH + 3O2 +2H2O.
Question 23.
Dilute solution of hydrogen peroxide cannot be concentrated by heating. How can it be concentrated ?
Answer:
Dilute solution of H2O2 cannot be concentrated because it decomposes much below its melting point.
2H2O2 → 2H2O + O2
1% H2O2 liberated from water is distilled at low pressure and concentrated to 30% by mass. Again it is distilled at low pressure upto 85%. Remaining water is freezed to obtain pure hydrogen peroxide.
Question 24.
For the manufacture of hydrogen peroxide by peroxides phosphoric acid is more useful than sulphuric acid. Why?
Answer:
H2SO4 acts as a catalyst in the decomposition of H2O2. Thus, in the manufacture of H2O2 by peroxides weak acids like H3PO4, H2CO3 etc. are more suitable than H2SO4.
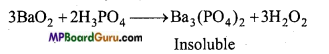
Question 25.
Ionic hydride of a basic metal exhibits effective covalent behaviour and it is unreactive towards oxygen and chlorine. It is used in the synthesis of other useful hydrides. Write the formula of this hydride. Write its reaction with Al2Cl6.
Answer:
It is ionic hydride LiH. Due to smallest size it shows covalent behaviour also. LiH is extremely stable. It is extremely unreactive towards oxygen and chlorine. It reacts with Al2Cl6 to form Lithium aluminium hydride.
8LiH +Al2Cl6 → 2LiAlH4 +6LiCl.
Question 26.
Why does hard water do not for lather readily with soap?
Answer:
In hard water bicarbonates, chlorides and sulphates of Ca and Mg are dissolved. Soaps are sodium salt of higher fatty acids e.g., sodium stearate (C17H35COONa). Salts of calcium and magnesium react and form precipitates of calcium and magnesium stearate.
C17H35COONa + M2+ → (C17H35COO)2 M + 2Na+
Until all the salts are precipitated, no lather is formed with soap and this soap is wasted.
Question 27.
Write four uses of hydrogen peroxide.
Answer:
Uses of hydrogen peroxide :
- It is an antiseptic, used for washing teeth, ears, wounds, etc.
- As an oxidizing agent in laboratory.
- It is used for bleaching hair, silk, wool, feathers, ivory, etc.
- As a rocket fuel (as propellant) by producing oxygen.
Question 28.
Write industrial method of preparation of hydrogen by water gas.
Answer:
Water gas is produced by passing steam over red hot coke.
![]()
Water gas is mixed with steam and heated at a temperature of 450 °C in presence of Fe2O3 as catalyst and chromic oxide as promoter when carbon monoxide gets oxidized to carbon dioxide.
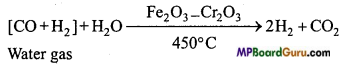
Question 29.
What is conductivity water? Write its use.
Answer:
Kohlrausch distilled water 42 times at low pressure in a Quartz container and obtained exteremely pure water. This is known as conductivity water. Some potassium permanganate is mixed in distilled water and it is again distilled in a strong glass retort. It is used in the determination of conductivity.
Question 30.
What is deuterium? Write its two uses.
Answer:
Deuterium is an isotope of hydrogen. It is called heavy hydrogen. Its nucleus consists of one proton and one neutron and one electron rotates around the nucleus. That means its atomic weight is 2 and atomic number is 1. It is represented as H12 or D. In common H2 gas it is presented 0.0156%. It was discovered by Unbrickwade and Murphy. Deuterium is obtained by the electrolysis of heavy water at cathode and O2 is obtained at anode.
![]()
Uses: 1. It is used to determine the reaction mechanism of chemical and biochemical reactions.
2. It is used in the artificial transmutation of element as a particle in the form of deuteron.
7N14+1D2→ BCl2+2He4.
Question 31.
Sodium reacts with dihydrogen to form crystalline ionic solid. This compound is non-volatile and non-conducting in nature. It reacts readily with water to form dihydrogen gas. Write the formula of this compound and its reaction with water. What will happen on electrolysing it in molten state?
Answer:
Sodium reacts with dihydrogen to form sodium hydride which is a crystalline ionic solid. ‘
2Na + H2 → 2Na++H–
It reacts readily with water to form H2 gas
2NaH + 2H2O →2NaOH+2H2
In solid state, electric current does not flow in NaH but in molten state it releases H2 at anode and Na at cathode.
![]()
Question 32.
Write the redox reaction between fluorine and water.
Answer:
Fluorine is a strong oxidizing agent. It oxidizes H2O to 02 and O3.
2F2(g) +2H2O(l) → O2(g) + 4H+(aq) +4F–(aq)
3F2(g) +3H2O(l) → O3(g)+6H+(aq) +6F–(aq)
Question 33.
Explain: HCl is a gas and HF liquid. Why?
Answer:
Size of F is smaller than Cl and its electronegativity is also more. Thus, it forms stronger H-bond than Cl. That is why HF is a liquid and HC1 a gas.
Question 34.
Write a chemical equation for the manufacture of D2O2.
Answer:
D2O2 is obtained by the action of BaO2 on water-soluble D2SO4.
BaO2 + D2SO4 → BaSO4 + D2O2.
Question 35.
Why can H2O2 not be stored for a long time?
Answer:
H2O2 contain peroxide bond. Due to the presence of this bond it is unstable and starts decomposing.
2H2O2 → 2H2O + O2
As a result H2O2 cannot be stored for a long time. This decomposition reaction is catalyzed by glass, therefore the decomposition becomes faster in glass bottle. To stop it, it is stored in a coloured waxy of layered bottle.
Question 36.
H2O2 is known as an antichlor. Why?
Answer:
In coloured substances in which bleaching is done by chlorine, some amount of chlorine is left remaining. This way to remove the remaining chlorine H202 is used. Due to this property, it is known as an antichlor.
Cl2+H2O2 → 2HCl + O2.
Question 37.
On boiling temporary hard water v .th slaked lime it becomes soft. Why?
Answer:
When temporary hard water is boiled with slaked lime then Ca and Mg bicarbonates present in hard water gets converted to insoluble bicarbonates which are removed by Alteration.
Ca(HCO3)2 +Ca(OH)2 → 2CaCO3 ↓+2H2O
Mg(HCO3)2 + Ca(OH)2 → MgCO3 + CaCO3 + 2H2O.
Question 38.
Saline hydrides are known to react with water violently producing fire. Can CO2, a well-known fire extinguisher, be used in this case. Explain.
Answer:
Saline hydrides (NaH, CaH2 etc.) react with water violently to produce metal oxide and dihydrogen.
Question 39.
If equal weight of liquid water and ice pieces are taken then density of ice is less than water. Why?
Answer:
In ice, water molecules are not denser as in liquid water. In the lattice structure of ice vacant voids are present due to which its volume becomes more and density less.
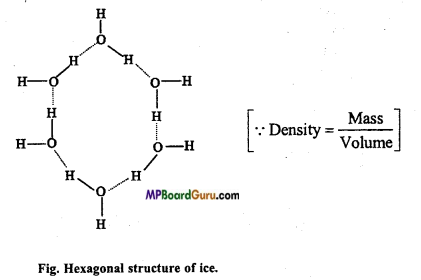
Question 40.
Explain one method of preparation of Hydrogen peroxide.
Answer:
Peroxide method: H2O2 is formed by the action of sodium peroxide on ice-cooled 20% H2SO4.
Na2O2 + H2SO4 → H2O2 + Na2SO4
On cooling, sodium sulphate separate in the form of crystals Na2SO4.5H2O which is filtered and 30% H2O2 solution is obtained.
Question 41.
In which compound oxidation state of hydrogen is negative?
Answer:
When hydrogen combines with lighter and extremely reactive metals like Na, K, Ca etc. then electrovalent hydrides are formed. In these electrovalent hydrides, oxidation state of hydrogen is negative. -1.
![]()
Question 42.
Dihydrogen (H2)- reacts with dioxygen (O2) to form water. When an isotope of hydrogen which has one proton and one neutron in the nucleus is reacted with oxygen, then write the name and formula of the product formed. Will the reactivity of both the isotopes with oxygen be same? Discuss your answer.
Answer:
Isotope of hydrogen whose nucleus has one proton and one neutron, is deuterium
(D, 12H).
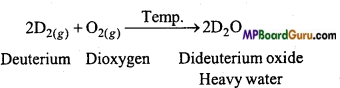
Reactivity of H2 and D2 is the different towards oxygen. D-D bond is stronger as compared to H-H bond. Thus, H2 is more reactive than D2.
Hydrogen Class 11 Important Questions Short Answer Type
Question 1.
Justify the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
Hydrogen is the first element placed in the periodic table. Its electronic configuration is 1s1. Like alkali metals, it can lose one electron and form H+ ion. Like halogens, it can gain one electron and form H– ion. Like alkali metals it forms oxide, halide and sulphide.
Though alternative to alkali metals its ionization enthalpy is high and under normal conditions it does not exhibit metallic behaviour. Actually in terms of ionization enthalpy it exhibits more similarity towards halogens. Like halogens it forms diatomic molecule, reacts with elements to form hydrides and various covalent compounds. Though, alternative to halogens its reactivity is very less.
Thus, on the basis of the above properties, hydrogen should be placed with alkali metals in Group-1 in the periodic table or with halogens in Group-17 in the periodic table. Although it shows similarity in properties with alkali metals and halogens it also show dis-similarities with them. Thus, it should be given a separate place in the periodic table.
Question 2.
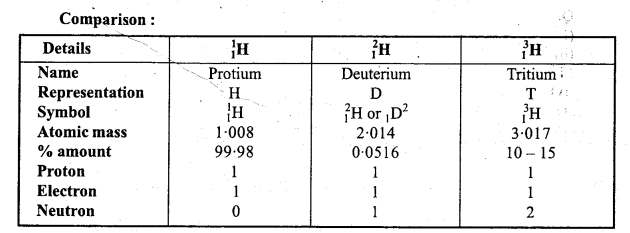
Write the name of isotopes of hydrogen and state what is the mass ratio of these isotopes ?
Answer:
Isotopes of hydrogen are Protium (11H), Deuterium (D or 12H) and Tritium (T or 3H )
Mass ratio of Protium, Deuterium and Tritium =1 : 2 : 3.
Question 3.
H2O2 is used for restoring the colour of lead paintings. Explain this state-ment.
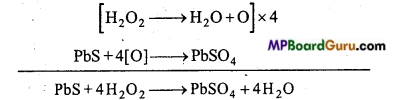
Ans. The lead painting contain white lead which is basic lead carbonate. The colour of these painting gradually becomes blackish due to the formation of PbS by the action of H2S of the atmosphere. As by the action of H2O2 lead sulphide is oxidized to lead sulphate, colour of lead paintings is restored.
![]()
Such lead paintings when washed with H2O2 become bright due to conversion of black PbS to white PbSO4.
Question 4.
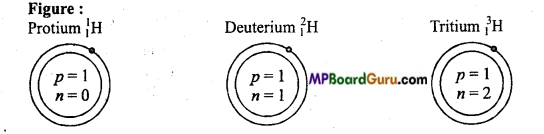
Draw the figure of all the isotopes of hydrogen and write their names.
Answer:
There are three isotopes of hydrogen :
- Normal hydrogen or Protium : 11H
- Heavy hydrogen or Deuterium: 12H
- Tritium: 31H.
Question 5.
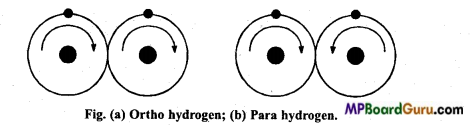
Explain ortho and para hydrogen.
Answer:
Only one proton is present in the nucleus of H atom and one electron rotates around it. Like electron, proton also spin around its one axis. If protons of two atoms of H2 molecule rotates in the same direction, then it is called ortho hydrogen, but when proton of two H atoms rotates in opposite directions, then it is called para hydrogen. Para hydrogen is more stable and general hydrogen is the para form.
Question 6.
Hard water is not used in boilers. Why?
Answer:
Hard water is not used in boilers because compounds of calcium and magnesium are deposited on internal sides of boiler. This coating is not conductor of heat, so wastes of fuel takes place. Due to this coating, boilers have to be heated very much for heating water.
At high-temperature Fe of water surface of boiler react with O2 of air and form Fe3O4. Sometimes hot water directly react with iron surface from the gaps between these coatings and form Fe3O4 and H2.
Hydrogen is volatile gas therefore there is risk for explosion. If MgCl2 is present in hard water, it react with water and form HCl. This acid is corrosive for boiler.
MgCl2 +H2O → Mg(OH)Cl + HCl.
![]()
Question 7.
How can production of dihydrogen obtained by ‘coal gasification’ be increased?
Answer:
Production of syngas or synthetic gas by coal is known as ‘coal gasification.
![]()
CO2 produced is separated by passing through sodium arsenite solution.
Question 8.
Describe the bulk preparation of dihydrogen by electrolytic method. What is the role of an electrolyte in this process?
Answer:
Hydrogen is obtained by the electrolysis of acidulated water by platinum electrodes.
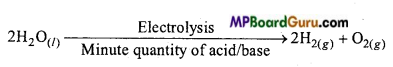
Role of electrolyte is the conductivity of water.
Question 9.
What happens when :
(i) Calcium hydride is reacted with water.
(ii) H2O2 is mixed in acidic KMnO4.
(iii) Alkaline solution of Potassium ferricyanide is reacted with H2.
(iv) Reaction of H2 with N2 in presence of Feat high temperature and at high pressure.
Answer:
(i) Calcium hydride reacts with water to form calcium hydroxide and hydrogen.
CaH2 +2H2O → Ca(OH)2 +2H2
(ii) H2O2 reacts with acidic KMnO4 and decourises it.
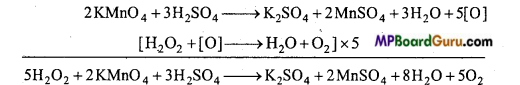
(iii) H2O2 reduces alkaline solution of potassium ferricyanide to potassium ferrocyanide.
2K3[Fe(CN)6]+2KOH+H2O2 → 2K4[Fe(CN)6]+2H2O+O2
(iv) At high temperature and high pressure in the presence of Fe as catalyst and Mo as promoter, H2 is reacted with N2, ammonia is formed.
![]()
Question 10.
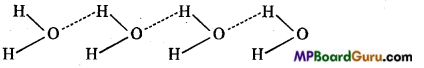
At normal temperature H2O is liquid while H2S is gas. Why?
Answer:
Electronegativity of oxygen is comparatively higher than the hydrogen so in H2O molecule, bonding electrons of O-H bond are attracted towards oxygen. Thus, partial positive charge (δ+) develops on hydrogen and partial negative (δ–) charge on oxygen.
When many water molecules are close to each other positively charged hydrogen form a weak bond with negatively charged oxygen of another atom. This type of bond is called hydrogen bonding. Due to hydrogen bonding many molecules are associated very close to each other. Therefore, water is in liquid state.
In H2S hydrogen bonding is not present because S is less electronegative than oxygen. Therefore, H2S is a gas.
Question 11.
Write the following reactions with H2O2 (Give only chemical equation):
(a) O3,
(b) PbS,
(c) KI,
(d) Cl2,
(e) H2S.
Answer:
(a) Reaction with O3: Water is formed and liberates oxygen.
2O2 + O3→ H2O + 2O2
(b) Reaction with PbS: PbSO4 is formed.
4H2O2 + PbS → PbSO4 + 4H2O
(c) Reaction with KI: Iodine is liberated.
2KI + H2O2 → 2KOH +I2
(d) Reaction with CI2: HCl is formed and oxygen is liberated.
Cl2 +H2O2 → 2HCl + O2
(e) Reaction with H2S: Hydrogen peroxide oxidizes H2S to S.
H2S +H2O2 → 2H2O +S.
Question 12.
Do you expect the carbon hydrides of the type (CnH2n+2) to act as lewis acid or base? Justify your answer.
Answer:
(CnH2n+2) type of carbon hydrides are electron-rich hydrides. They contain sufficient electrons to form covalent bond. Therefore, they neither behave as Lewis acid nor as Lewis base.
Question 13.
What do you understand by the term “non-stoichiometric hydrides”? Do you expect this type of hydrides to be formed by alkali metals? Justify your answer.
Answer:
These hydrides are formed by cf-block elements (except metals of group 7, 8 and 9) and f-block elements. Hydrides are always non-stoichiometric i.e. are hydrogen deficient. In these hydrides, hydrogen lie in the interstitial sites.
Example : LaH2.87, YbH2.55,TiH15-1.8,PdH0.6- 0.8etc.
This type of hydrides cannot be obtained by alkali metals. By alkali metals ionic or saline hydrides are obtained. These are stoichiometric. Alkali metals are highly electropositive, thus they transfer electron to H-atom and form H– ion. These H– ion occupy the interstitial voids.
Question 14.
Complete the following equations :
(i) PbS(s) + H2O2(aq) →
(ii) CO(g) + 2H2(g)![]()
Answer:
(i) PbS(s) + 4H2O2(aq) → PbSO4 + 4H2O
(ii) ![]()
Question 15.
What is water gas? Write its composition and use.
Answer:
Water Gas: This gas is a mixture of carbon monoxide and hydrogen. On passing water vapour on red hot coke mixture of carbon monoxide and hydrogen (water gas) is obtained.
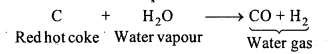
Composition : H2 = 49%, N2 = 4%, CO = 44%, CO2 = 2.7%, CH4 = 0.3%.
Use :
- As a fuel in furnaces,
- In the presence of carburetted water gas,
- In the manufacture of ammonia,
- In providing light with unsaturated hydrocarbons.
Question 16.
Hardness of water is of how many types? How is temporary hardness removed?
Answer:
Water which contains soluble bicarbonates, sulphates and chlorides of Ca and Mg is hard water. Hardness of water is of two types :
(i) Temporary hardness,
(ii) Permanent hardness.
Note:
Hardness of water is due to the presence of soluble bicarbonates, sulphates, chlorides, salts of calcium and magnesium.
1. Temporary hardness: When hardness of water is removed by boiling or by simple methods then it is known as temporary hardness. Temporary hardness is due to bicarbonates of Ca and Mg.
2. Permanent hardness: If hardness is not removed by boiling or by simple methods, it is known as permanent hardness. It is due to chlorides and sulphates of Ca and Mg.
There are two methods for the removal of temporary hardness :
1. By Boiling: On boiling soluble bicarbonate of Ca and Mg gets converted to insoluble carbonates and gets precipitated.
Ca(HCO3)2 → CaCO3 + H2O + CO2
Mg(HCO3)2 → MgCO3 + H2O + CO2
2. Clark’s method: Temporary hard water is mixed with definite amount of slaked lime when soluble bicarbonate converted to insoluble carbonates and gets precipitated.
Ca(HCO3)2+Ca(OH)2 → 2CaCO3+2H2O
Mg(HCO3)2 +Ca(OH)2 → MgCO3 +CaCO3 + 2H2O.
Question 17.
Draw Lewis structure of hydrogen peroxide.
Answer:
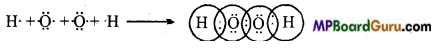
Question 18.
How do you expect the metallic hydrides to be useful for hydrogen storage? Explain.
Answer:
In metallic hydrides, hydrogen is absorbed in the form of H-atom. In transition metals, adsorption of hydrogen is used for the storage of hydrogen. Metals like Pd, Pt can accomodate bulk volume of hydrogen. This property has strong possibility of hydrogen storage and is used as an energy source. Hydrides on heating give hydrogen and the metal.
Question 19.
How does atomic hydrogen or oxy-hydrogen torch function for cutting and welding purposes? Explain.
Answer:
Atomic hydrogen and oxy-hydrogen torches find use for cutting and welding purposes. Atomic hydrogen atoms produced by dissociation of dihydrogen with the help of electric arc are allowed to recombine on the surface to be welded to generate the temperature of 4000 K.
![]()
Question 20.
Compare the structures of H2O and H2O2.
Answer:
In water, oxygen is sp3 hybridized. Due to stronger lone pair-lone pair repulsion than bond pair-bond pair repulsion, HOH bond angle reduces from 109.5° to 104.5° due to which water has a bent shape. It is strongly polar molecule. Structure of hydrogen peroxide is non-polar.
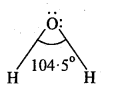
Dipole moment value of H2O2 indicates that all the atoms of H2O2 do not lie in a plane. Structure of H2O2 can be compared to an open book at 94°C. In it H-O-H bond angle is 91°.
Question 21.
Explain structure of hydrogen peroxide.
Answer:
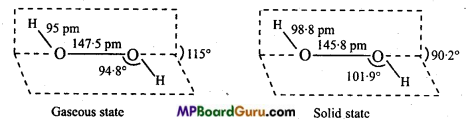
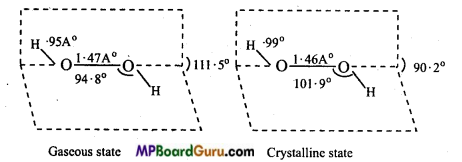
Dipole moment of H2O2 is 2.1 D, it indicates that structure of H2O2 is non-planar. By X-rays and other physical methods it is known that structure of H2O2 is like open book. In gaseous state planes form an angle of 111.5°. In the axis, there are two oxygen atoms and one hydrogen in each plane.
The H-O and O-O bond lengths are 0.95 Å and 1.47 Å respectively. There is a slight change in the crystalline state due to hydrogen bonding. The angle between the planes is 90.2, angle O-O-H = 101.9°, bond length H-O = 0.99 A and O-O is 1.46Å.
According to modem and latest belief probably both forms occur in equilibrium in aqueous solutions involving ionisation which explains the feeble acidic nature of hydrogen peroxide.
Question 22.
Explain that H2O2 acts as an oxidizing as well as reducing agent.
Answer:
H2O2 liberates oxygen atom easily, so it is strong oxidising agent, but when it reacts with other oxidising agent, it easily extracts oxygen atom from them hence it possesses reducing property too.
(a) Oxidising nature : 2KI + H2O2 → 2KOH +I2
Na2SO3 + H2O2 → Na2SO4 + H2O
H2S + H2O2 → 2H2O + S
(b) Reducing nature:
H2O2 + O3 → H2O + 2O2
PbO2 + H2O2 → PbO + H2O + O2
Cl2 + H2O2 → 2HCl + O.
Question 23.
What is calgon ? How is hardness of water removed by its help?
Answer:
Calgon process: It is a beneficial and industrial method of softening of water. Calgon is poly hexa metaphosphate. Its sodium salt reacts with calcium and magnesium salts forming soluble complex compound by which calcium and magnesium are not left in the form of ions.
Na2[Na4(PO3)6] + CaSO4 → Na2[CaNa2(PO3)6] + Na2SO4
Na2[CaNa2(PO3)6] + CaSO4 → Na2[Ca2(PO3)6] + Na2SO4.
![]()
Question 24.
What do you understand by the term ‘autoprotolysis’ of water? What is its significance?
Answer:
Autoprotolysis of water means, self ionisation of water.
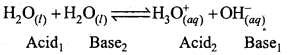
Due to autoprotolysis, nature of water is amphoteric i.e. it reacts both as an acid as well as a base. ‘
For example:
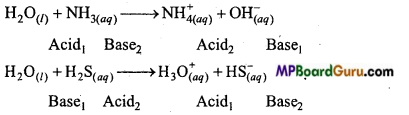
Question 25.
Consider the reaction of water with F2 and suggest, in terms of oxidation and reduction, which species are oxidized/reduced.
Answer:
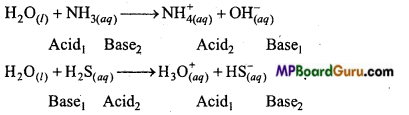
In these reactions, water acts as a reducing agent. Thus, it itself oxidizes to oxygen or ozone. Fluorine acts as an oxidizing agent and itself gets oxidized to F– ion.
Question 26.
How does H2O2 behave as a bleaching agent? Write.
Answer:
Due to the production of nascent oxygen H2O2 acts as a bleaching agent.
H2O2 → H2O+O
Coloured substance + [O] → Colourless substance
It oxidizes silk, hairs, clothes, paper pulp, wool, oil, fats etc.
Question 27.
How many types of hydrates are there? Explain with example.
Answer:
Water combines with various metal salts and forms hydrate. It is of three types :
1. Complexion cationic water: In complexion, it is present in cation forming ligand with the central metal.
Example: Hexa aqua chromium (III) chloride [Cr(H2O)6]Cl3
2. Complexion oxyanion water: In complex ion water molecule is present with oxyanion.
CuSO4 +5H2O → [CU(H2O)4]2+ + [SO4 (H2O)]2-
3. Crystalline water: In crystal lattice, water molecule is present in the interstitial voids between the molecules which is known as water of crystallization. Some metal salts lose the crystallized water on standing. This is known as efflorescence. Various substances absorb water from air on keeping in air.
Question 28.
Describe permutit process or zeolite process for the removal of hardness of water.
Answer:
Permutit process or Zeolite process: The water is passed over such substances which readily replace the Ca2+ and Mg2+ ions of hard water by sodium ions. The water does not become hard in presence of sodium salts. Of course too much of sodium salts also make it hard just as in seawater. The water thus obtained is soft. These products are called as zeolites. Zeolite is a technical name given to certain hydrated silicates of aluminium and sodium. It is also known as permutit. It is obtained by fusing sodium carbonate with alumina and silica.
Na2CO3+Al2O3+2SiO2 → Na2Al2Si2O8+CO2
Na2Al2Si2O8 is known as sodium zeolite and Al2Si2O8 is zeolite and can be represented by Z. Sodium zeolite reacts with chlorides, sulphates of Ca and Mg as given below and hardness is removed.
CaCl2 +Na2Z → CaZ + 2NaCl
MgSO4 + Na2Z → MgZ + Na2SO4
Reactions have been given by representing sodium zeolite as Na2Z.
After sufficient use permutit gets exhausted and cannot be used further. It is regenerated and made usable by passing 10% solution of sodium chloride.
CaZ + 2NaCl → CaCl2+Na2Z
MgZ + 2NaCl → MgCl2 + Na2 Z.
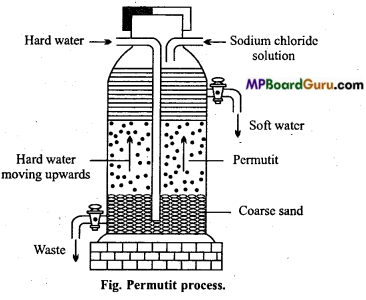
Question 29.
What is meant by ‘demineralized’ water and how can it be obtained?
Answer:
Water which is free from all soluble mineral salts is called demineralised water. To obtain demineralized water, water is flown through cation exchange resin and anion exchange resin.
In cation exchange Ca2+, Mg2+, Na+ and other cations present in water are exchanged
by H+ ions and separated whereas in anion exchange Cl–, HCO3, SO4” etc. anions are exchanged by OH– ions and separated.
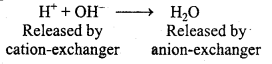
Question 30.
What properties of water make it useful as a solvent? What types of compound can it (i) dissolve and (ii) hydrolyse. ,
Answer:
Due to high dipole moment and high dielectric constant water is useful as a solvent.
(i) It can dissolve ionic compounds and these covalent compounds in which H-bond is found with water. (Like ethanol, sugar, glucose etc.)
(ii) Water can hydrolyse various metallic and non-metallic oxides, hydride, carbide, phosphide and other salts.
For example:
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
CaH2(s) + 2H2O(l) → Ca(OH)2(aq)+2H2(g)
Al4C3(s) + 12H2O(l) →4Al(OH)3 + 3CH4.
![]()
Question 31.
Describe the usefulness of water in biosphere and biological systems.
Answer:
Major portion of body of all living organisms is made up of water. Human body contains about 65% part and some plants contain about 95% part water. Water is an important compound for the survival of all living organisms. Compared to other liquids, specific heat of water, thermal conductivity, surface tension, dipole moment and dielectric constant of water is very high.
Due to these properties role of water in the biosphere is very important. For maintaining the body temperature and normal climatic conditions, heat of vaporization and high specific heat of water is responsible. It acts as a best solvent for the transport of molecules in the metabolism of plants and animals. Water is also necessary for photosynthesis in plants in which O2 is released in the environment.
![]()
Question 32.
Describe the structure of water molecule and also explain that water is a good solvent.
Answer:
In water molecule, 2H atoms and one oxygen atom are linked with covalent bond. Central atom of water molecule is sp3 hybridised and its shape is tetrahedral. In H2O molecule two sp3 – s sigma bonds and remaining are two lone pair of electrons. Due to repulsion of lone pair-lone pair electrons, bond angle is 104° 27′ in place of 109° 28′ (angle of regular tetrahedron).
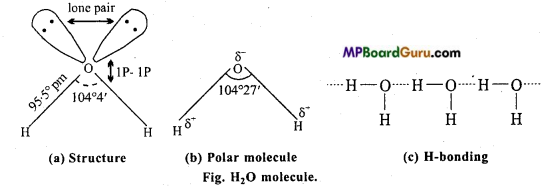
Properties of good solvent: Water is a good solvent because water molecule is polar and between its molecules, hydrogen bonding exist. Due to polarity and high dielectric constant ionic compounds and polar covalent compounds are soluble in water. Due to hydrogen bonding those compounds are also soluble which have H-bonding or form hydrogen
bonds with water molecules.
Question 33.
Explain the preparation of hydrogen peroxide from barium peroxide.
Answer:
Preparation of pure hydrogen peroxide :
1. By dilute sulphuric acid: By the action of dil. H2SO4on hydrated barium peroxide H< sub>2O2 is formed. Precipitate of BaSO4 is also formed which is filtered out.
BaO2.8H2O + H2SO4 → BaSO4 ↓+H2O2 +8H2O
2. By the action of Carbon dioxide on Barium peroxide: A paste of hydrated barium peroxide is made in freezed water and carbon dioxide gas is slowly passed through it. Precipitate of barium carbonate is filtered and separated.
BaO2 + CO2 + H2O → BaCO3 + H< sub>2O2.
Question 34.
How is hydrogen peroxide concentrated?
Answer:
Hydrogen peroxide solution obtained by any method is dilute, it is Concentrated at low temperature and low pressure because it decomposes on heating at normal pressure.
(a) Vaporization: By vaporizing the H2O2 at 70°C on a water bath, 45% H2O2 solution is obtained.
(b) Distillation at low pressure: 45% H2O2 solution is distilled at 15 mm pressure and 60°C temperature. By this about 90% of H2O2 solution is obtained.
(c) Now, this solution is evaporated at vacuum desicators over concentrated H2SO4 and 99% cone. H2O2 is obtained.
(d) Crystallization: This solution is crystallized by freezing mixture of solid CO2, ether and crystals of H2O2 are obtained. It is melted and pure H2O2 liquid is obtained.
Question 35.
How is heavy water manufactured? Write its properties.
Answer:
By the fractional distillation of ordinary water : About one part of heavy water is present in 6000 parts of ordinary water. Heavy water can be separated by the fractional distillation of ordinary water. Boiling point of heavy water is higher than that of ordinary water. Therefore, on fractional distillation of ordinary water, ordinary water is first distilled and heavy water remains back as residue.
Physical properties: Heavy water is colourless, odourless, tasteless liquids. Its freezing point is 3.8°C and boiling point is 101.4°C.
Chemical properties :
1. Electrolysis: By the electrolysis ‘of heavy water in the presence of an electrolyte, deuterium is obtained at cathode and 02 gas is obtained at anode.
2D2O → 2D2 + O2 ,
2. Reaction with nitrides: By the reaction of heavy water with nitrides, heavy ammonia is formed.
Mg3N2 + 6D2O → 3Mg(OD)2 + 2ND3 Heavy ammonia.
![]()
Question 36.
Density of water is maximum at 4°C. Why?
Answer:
When ice melts to liquid state, the cage like structure present in ice breaks. Number of hydrogen bond decreases and due to breakage of H-bond the vacant space in the structure of ice leads to arrangement of water molecules, the water molecules come very close, as a result density of water increases, but beyond 4°C temperature kinetic energy of water molecules increases as a result water molecules move away. This results in the in¬crease in volume and decrease in density.
Question 37.
Name the classes of hydrides to which H2O, B2H6 and NaH are related.
Answer:
H2O: Covalent or molecular hydride (Electron rich hydride)
B2H6: Covalent or molecular hydride (Electron deficient hydride)
NaH: Ionic or Saline hydride.
Hydrogen Class 11 Important Questions Long Answer Type
Question 1.
Discuss the theory and method of conversion of hard water to soft water by synthetic ion exchange.
Answer:
Synthetic ion exchange resin method is as follows :
1. Cation-exchange resin: These resins are -SO3H group giant organic molecules insoluble in water. These are treated with NaCl to convert into R-Na. Resin RNa exchanges the Mg2+ and Ca2+ ions and converts it to soft water.
2RNa(s)+M2+ (aq) → R2M(s) +2Na+(aq) (M = Ca2+ or Mg2+)
On passing aqueous NaCl solution, it is regenerated. To obtain pure demineralized and deionized water, the water obtained above is again passed through cation-exchanger and anion-exchanger resin.
In cation-exchanger process H+ is exchanged by Na+, Ca2+, Mg2+ etc. ions present in water.
2RH(s) + M(2+)(aq) ⇌ MR2(s) + 2H+(aq)
(Cation exchange resin in the form of H+)
In this process protons are produced and water becomes acidic.
2. Anion-exchange resin: In this process OH– are exchanged by Cl–, HCO–3, SO-24 ions present in water.
![]() is substituted ammonium hydroxide anion resin.
is substituted ammonium hydroxide anion resin.
![]()
This way OH– ion released, neutralizes H+ ions. At the end, cation and anion exchange resin are treated with dilute acid and dilute alkaline solutions and regenerated.
Question 2.
Discuss the position of hydrogen in the periodic table.
Answer:
Position of hydrogen in the periodic table: Hydrogen is placed in the first group and first period of periodic table. It resembles both the alkali metal of group 1 and the halogens of group 17. It also shows some similarities with carbon which is placed in group 14. But considering all the points, its position in group 1 of the periodic table is most suitable.
Resemblance with alkali metals: Hydrogen is placed along with alkali metals because:
(i) s-block element: Hydrogen is s-block element and its electronic configuration is 1s1.
(ii) Electronic configuration: It has same number of electrons in its Outermost orbit like Na and K which are alkali metals of first group.
H = 1;Na = 2,8,1;K = 2,8,8,1.
(iii) Valency: Valency of hydrogen and alkali metals is one.
(iv) Formation of cation : Like alkali metals it loses one electron to form a cation.
H – e– → H+
Na-e– →Na+
(v) Electropositive nature: Like alkali metals it is electropositive in nature. It reacts
with electronegative elements like sulphur, oxygen and chlorine etc. .
H2 +S →H2S
2Na + S → Na2S
(vi) Oxidation number: Like alkali metals its oxidation number in salts is + 1.
(vii) Reducing properties : Like alkali metals hydrogen is also a reducing agent.
Resemblance with halogens :
(i) Electronic configuration : Like halogens it is also in short of one electron to the inert gas of adjacent group.
H = 1 He = 2
Cl =17 Ar = 18
(ii) Ionisation potential: Ionisation potential of hydrogen is quite close to ionisation potential ofhalogens but ionisation of alkali metals is very low.
H → 13.5eV, Cl → 13.0eV,Na → 5.1eV.
Where, eV = potential in electron volt.
(iii) Atomicity : Hydrogen molecule is diatomic like halogen molecules. The value of \(\frac{\mathrm{C}_{p}}{\mathrm{C}_{v}}\) in both H2 and halogens is = 1.4.
H – H or H2 Cl – Cl or Cl2
(iv) Non-metallic nature : Like halogens (F, Cl, Br and I) hydrogen is a non-metal and is a gas.
(v) Combination with metals: Both hydrogen and halogens react with metals.
![]()
(vi) Formation of covalent compounds : Both hydrogen and halogens react with carbon, silicon and nitrogen to form covalent-compounds.

Special features of hydrogen nature: In some properties hydrogen also show differences with alkali metals and halogens both as :
(i) Oxide of hydrogen is neutral while oxide of halogen is acidic and oxides of alkali metals are basic in nature.
(ii) Outermost orbit is half-filled like group four elements.
(iii) Its atomic structure is different with all other elements.
On the basis of these facts it is thought that special position should be provided for hydrogen in periodic table but due to electronic configuration of 1s1 and its more resemblance, its place is supposed in group first with alkali metals.
Question 3.
Do you expect different products in solution when aluminium (III) chloride and potassium chloride treated separately with (i) Normal water, (ii) Acidified water and (iii) Alkaline water? Write equations wherever necessary.
Answer:
AlCl3 is a salt of weak base Al(OH)3 and strong acid HCl. Thus on being reacted with water, it hydrolyses.
AlCl3(s) + 3H2O(l) → Al(OH)3 + 3H–(aq) +3Cl–(aq)
Its aqueous solution is of acidic nature. H+ ion present in acidified water reacts with Al(OH)3 to produce Al3+(aq) ion and water. Thus, in acidified water, AlCl3 exists in the form
Al3+(aq) and Cl–(aq) ions. In alkaline water AlCl3 produces the following products :
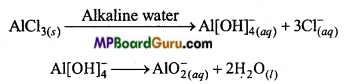
KCl is a salt of strong acid HCl and strong base KOH. It does not hydrolyse in normal water. It hydrolyses, in water to give K+(aq) and Cl–(aq) ion.
![]()
Aqueous solution of KCl is neutral. Thus, in acidified water or in alkaline water, its ion does not react.
![]()
Question 4.
Describe laboratory method of preparation of pure and dry hydrogen gas.
Answer:
Preparation of hydrogen (laboratory method) : Hydrogen is prepared in laboratory by action of dil. H2SO4 on granulated Zn. Zn is taken into Woulf’s bottle fitted with Thistle funnel and a outlet tube.
Hydrogen formed by this method is collected over water by displacement method.
Zn + H2SO4 → ZnSO4+H2 ↑
Purification of hydrogen : Hydrogen prepared by this method contains following impurities:
(a) Arsine (AsH3) and phosphene (PH3)
(b) H2S
(c) SO2,CO2 and oxides of nitrogen (NO2)
(d) Water vapours.
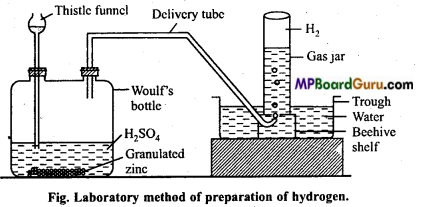
In order to remove these impurities, gas is passed in a series of U-tubes containing different solutions.
- Passed in tube filled with AgNO3 solution to absorb AsH3 and PH3.
- Passed in the tube containing lead nitrate solution to remove H2S.
- Passed in the tube filled with cone. KOH solution to remove SO2, CO2 and NO2.
Question 5.
What is demineralized water? How is it obtained? Or, Explain ion exchange method of softening of hard water with diagram.
Answer:
Water obtained by the removal of cation and anion present in it, is called demineralized water. It is good like distilled water. For this two types of ion exchange resins are used.
1. Cation exchange resin: It is an acidic resin represented by RSO3H. On flowing hard water over it, Ca2+ and Mg2+ all cations present in hard water displace hydrogen and occupy its place.
2RSO3H + CaCl2 → (RSO3)2Ca +2H+ + 2Cl–
2RSO3H + MgSO4 → (RSO3)2 Mg+2H+ + SO2-4
This H+ ion makes the water acidic.
2. Anion exchange resin: It is a basic resin which is represented by RNH2. It reacts with water to form RNH3OH.
R-NH2+H2O → RNH3OH–
After cation exchange resin, when water is passed over this resin, then the anions like chloride, sulphates liberates the OH~ ion of the resin.
RNH3OH + Cl– →RNH3Cl + OH–
2RNH3OH + SO2-4 → (RNH3)2SO4 + 2OH–
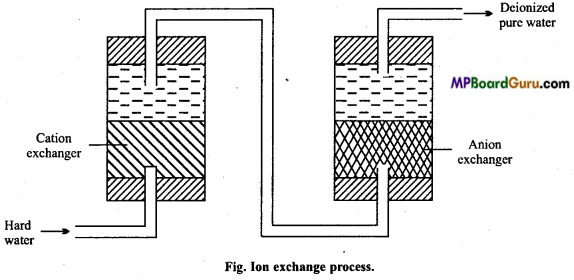
This OH– ion mixes with water and neutralizes the excess H+ present in it.
H+ +OH– →H2O
This way, water coming out through the other chamber is free from all types of cation, anion, acidity and basicity.
Question 6.
What are hydrides? Explain the different types of hydrides with example.
Answer:
When hydrogen combines with certain metals or non-metals (except inert gases) then the compounds formed are called hydrides. These hydrides are of three types according to the nature of chemical bond :
1. Ionic hydride: This type of hydrides are formed when hydrogen combines with strong electropositi ve elements. These hydrides are of ionic nature.
2Li + H2 →2LiH
2 Na + H2 →2NaH
These are also known as Saline hydrides.
Properties :
- These are generally non-volatile solid substances.
- They are white crystalline solids. Their crystal structure is unit ion.
- Their boiling and melting points are high.
- Density of these hydrides is less than their metals.
- They are conductors of electricity.
- Aqueous solution of ionic hydrides are basic.
NaH + H2O →NaOH + H2 - Atmospheric oxygen oxidizes and converts them to oxides.
CaH2+O2 →CaO + H2O
Uses :
1. As a reducing agent,
2. As a solid fuel.
II. Covalent hydrides: When hydrogen combines with p and s-block elements like Be and Mg then covalent hydrides are formed because of less difference in their electronegativity. These are also known as molecular hydrides.
Properties :
- Covalent hydrides are volatile compounds of low melting and boiling points.
- These hydrides are weak or bad conductors of electricity.
- van der Waals’ force is present between their molecules.
- According to electronegativity difference, their aqueous solution are acidic or alkaline.
- These are electron deficient compounds.
- On moving from top to bottom in .the group, stability of their hydride decreases.
III. Metallic hydrides: d-block elements and Be, Mg of s-block combine with hydrogen to form metallic hydrides. These are also known as interstitial hydrides because hydrogen gets arranged in the interstitial voids between the metal atoms.
Properties :
- These are hard and possess metallic lustre.
- They are good conductors of heat and electricity.
- Their density is less than the metals from which they are formed.
- These are unsymmetrical.
- They adsorb sufficient amount of hydrogen on their surface. This is known as adsorption of hydrogen.
Question 7.
Complete the following equations :
(i) H2(s)+MmOo(s) ![]()
(ii) CO(g) + H2(g) 
(iii) C3H8(g) + 3H2O(g) 
(iv) Zn(s) + NaOH(aq) ![]()
Answer:
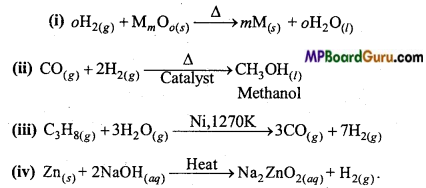
Question 8.
How is D2O obtained from water? Describe the different physical properties of D2O and H2O. Give minimum three reactions of D2O in which hydrogen is exchanged by deuterium.
Answer:
(a) Heavy water D2O is produced by the electrolysis of water.
(b) Physical properties:
(i) D2O is a colourless, odourless, tasteless liquid. At 11.6°C its density is maximum = 1.1071 gm L1 (Water at 4°C).
(ii) As compared to normal water solubility of salts in heavy water is less because it is more viscous than normal water.
(iii) Value of nearly all physical constants of D2O is comparatively more than that of water. This is due to stronger H-bond in D2O than D2O due to higher nuclear mass of D2O than H2O.
(c) Reactions of exchange of hydrogen by Deuterium:
NaOH + D2O → NaOD + HOD
HCl + D2O → DCl + HOD
NH4Cl + D2O → NH3DCl
Question 9.
Calculate the concentration of five-volume
Sol. 5 volume H< sub>2O2 solution means at N.T.P. by the decomposition of 1L of 5 volume sub>2O2,5L-1 O2 is obtained.
![]()
∵ At N.T.P. 22.4 L, 02 is obtained by = 68g H< sub>2O2
∴ At N.T.P. 5L, O2 will be obtained by = \(\frac{68 \times 5}{22 \cdot 4} \) g = 15.17g =
15gH< sub>2O2
But, at N.TP. 5L O2 is obtained by = 1L of 5 volume H< sub>2O2
∴ Concentration of H< sub>2O2 solution = 15g L-1
or Percent concentration of H< sub>2O2 solution =\(\frac{15}{100} \times 100\) = 15%
Question 10.
What weight of hydrogen peroxide will be present in 2L volume of 5 molar solution? Determine the amount of oxygen released by the dissociation of 200 ml of this solution.
Solution:
(i) Molar mass of H< sub>2O2 = 34g mol-1
Thus, amount of H< sub>2O2 present in 1L of 1M H< sub>2O2 solution = 34g
∵ Amount of H< sub>2O2 present in 2L of 5M H< sub>2O2 solution = 34 × 5 × 2 = 340g
(ii) Amount of H< sub>2O2 in 0.2L (or 200 ml) of 5M H< sub>2O2 solution
= \(\frac{340 \times 0 \cdot 2}{2}\) = 34 g H< sub>2O2
![]()
∵ Amount of O2 obtained by decomposition of 68g H< sub>2O2 = 32g .,
∴ Amount of O2 obtained by decomposition of 34g H< sub>2O2 = = 16g.
![]()
Question 11.
Rohan heard the instructions of a lab assistant for safe storage of a specific chemical. Keep it in dark, mix a small amount of urea in it, and keep it away from dust. This chemical behaves as an oxidizing as well as reducing agent in both acidic and alkaline medium. This chemical is used as pollution controller both in domestic and industrial field.
(i) Write the name of this chemical.
(ii) What precautions should be taken for its storage?
Answer:
(i) Name of the chemical is hydrogen peroxide, H< sub>2O2. It acts as an oxidizing as well as reducing agent in both acidic and alkaline medium.
(ii) By the presence of small amount of alkali present in light and dust, dissociation of H< sub>2O2 is catalyzed. That is why it is stored in glass or plastic container sealed with waxy layer in dark. To control its oxidation, urea is mixed in it as a negative catalyst.
![]()
Question 12.
Complete the following reactions :
(i) PbS(s) + H< sub>2O2(aq) →
(ii) MnO–4(aq) + H< sub>2O2(aq) →
(iii) CaO(s)+H2O(g) →
(iv) AlCl3(g) + H2O(l) →
(v) Ca3N2(s)+H2O(l) →
Classify above reactions as :
(a) Hydrolysis,
(b) Redox,
(c) Hydration.
Answer:
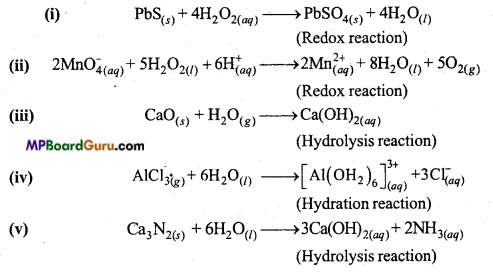
Question 13.
What do you understand by the following steps :
(i) Hydrogen economy
(ii) Hydrogenation
(iii) Syngas
(iv) Water-gas shift reaction
(v) Fuel cell.
Answer:
(i) Hydrogen economy: Main use of hydrogen and possibilities in the near future, is its use as pollution-free (clean) fuel. Basic principle of hydrogen economy is the transport and storage of energy as liquid hydrogen or gaseous hydrogen.
(ii) Hydrogenation : Combination of hydrogen in a double or triple bonded organic compound in the presence of a catalyst is called hydrogenation. Hydrogenation of vegetable oil in presence of Nickel catalyst forms edible fats (margarine and vanaspati ghee).
![]()
(iii) Syn gas: Mixture of CO and H2 is called syngas or synthetic gas or water gas. Syngas is obtained by the reaction of hydrocarbon or coke with steam at high temperature in the presence of catalyst.
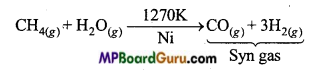
Now a days, ‘Syn gas’ is produced from sewage, sawdust, scrap wood, newspapers etc. The process of producing syngas from coal is called ‘coal gasification’.
(iv) Water-gas shift reaction: To increase the quantity of dihydrogen in water gas CO is treated with steam at 500°C in the presence of iron chromate as catalyst.

This reaction is known as water gas shift reaction.
(v) Fuel cell: In fuel cell, energy produced by the combustion of fuel is directly converted to electrical energy. Hydrogen-oxygen cell is the example of fuel cell. It does not produce any pollution. Fuel cell produces electricity with 70-85% conversion ability
Hydrogen Class 11 Important Questions Objective Type
1. Choose the correct answer:
Question 1.
Which of the following metal adsorb H2:
(a) Zn
(b) Pb
(c) Al
(d) K.
Answer:
(b) Pb
Question 2.
Radioactive isotope of hydrogen is known as:
(a) Protium
(b) Deuterium
(c) Heavy hydrogen
(d) Tritium.
Answer:
(d) Tritium.
Question 3.
Freezing point of heavy water is:
(a) 0°C
(b) 38°C
(c) 38°C
(d) -0.38°C.
Answer:
(b) 38°C
Question 4.
1 ml of H2O2 at N.T.P. gives 10 ml O2. This is:
(a) 10 volume H2O2
(b) 20 volume H2O2
(c) 30 volume H2O2
(d) 40 volume H2O2.
Answer:
(a) 10 volume H2O2
![]()
Question 5.
The least abundant isotope of hydrogen:
(a) 1H
(b) 2D
(c) 3 T
(d) (a) and (b).
Answer:
(c) 3 T
Question 6.
Heavy water is obtained :
(a) By boiling water
(b) By fractional distillation of heavy water
(c) By continuous electrolysis of water
(d) By heating H2O2.
Answer:
(c) By continuous electrolysis of water
Question 7.
Ortho and Para hydrogen differ by:
(a) Atomic number
(b) Atomic mass
(c) Electron spin
(d) Nuclear spin.
Answer:
(d) Nuclear spin.
Question 8.
Temporary hardness of watef can be removed :
(a) By boiling
(b) By adding CaCl2
(c) By adding CaCO3
(d) By adding Ca(OH)2.
Answer:
(a) By boiling
(b) By adding CaCl2
Question 9.
H2O2 solution:
(a) Turn red litmus blue
(b) Turn red litmus white
(c) Turn blue litmus red
(d) Turn blue litmus white.
Answer:
(b) Turn red litmus white
(d) Turn blue litmus white.
Question 10.
Which hydride do not exist in simple ratio:
(a) Ionic
(b) Molecular
(c) Interstitial
(d) All.
Answer:
(c) Interstitial
Question 11.
Is nO2 an example of ionic hydride:
(a) LiH
(b) CaH2
(c) CsH
(d) GeH2.
Answer:
(c) CsH
Question 12.
In H2O2 molecule H-O-O bond angle is :
(a) 99.5°
(b) 95.9°
(c) 97°
(d) 100.5°.
Answer:
Question 13.
Number of neutrons in heavy hydrogen atom is :
(a) 0
(b) 2
(c) 3
(d) 1.
Answer:
(d) 1.
![]()
Question 14.
Acts as a fuel for rocket:
(a) N2 + O2
(b) H2 + O2
(c) O2 + Ar
(d) H2 + N2.
Answer:
(b) H2 + O2
Question 15.
Density of water is more than ice :
(a) Due to hydrogen-bond
(b) Due to dipole attraction
(c) Due to polar-chaige polar
(d) Charged polarity.
Answer:
(a) Due to hydrogen-bond
Question 16.
Which of the following is used in calgon process :
(a) Sodium poly metaphosphate
(b) Hydrated sodium aluminium silicate
(c) Cation exchange resin
(d) Anion exchange resin.
Answer:
(a) Sodium poly metaphosphate
Question 17.
Reagent which is used to determine the hardness of water :
(a) Oxalic acid
(b) Disodium salt EDTA
(c) Sodium citrate
(d) Sodium thiosulphate.
Answer:
(b) Disodium salt EDTA
Question 18.
Hydrogen does not reduce :
(a)CuO
(b) Fe2O3
(c) SnO2
(d) Al2O3.
Answer:
(d) Al2O3.
Question 19.
Write CO2, H2O and H2O2 in increasing order of their acidity :
(a) CO2 < H2O2 < H2O
(b) H2O < H2O2 < CO2
(c) H2O < H2O22 > CO2
(d) H2O2 > CO2 > H2O.
Answer:
(b) H2O < H2O2 < CO2
Question 20.
Acts as a moderator in nuclear reactor :
(a) Hard water
(b) Heavy water
(c) Non-ionic water
(d) Mineralized water.
Answer:
(b) Heavy water
2. Fill in the blanks:
1. Hydrogen is released by the action of cone, solution of ………………….. on aluminium.
Answer:
NaOH
2. By the electrolysis of fiised NaH ………………….. gas is released at the anode.
Answer:
H2
3. Calgon is the commercial name of ………………….. .
Answer:
Sodium hexametaphosphate
4. In the reaction of F2 and H2O, H2O acts as a ………………….. .
Answer:
Reducing agent
5. By the action of water on calcium phosphide ………………….. is formed.
Answer:
Phosphine
6. Oxidized water is known as ………………….. .
Answer:
H2O2
7. ………………….. water give less lather with soap.
Answer:
Hard
8. Heavy water is mainly used in ………………….. .
Answer:
Nuclear reactors
9. Hardness of water is due to salts of ………………….. and ………………….. .
Answer:
Ca, Mg
10. ………………….. acts as tracer in the study of biological processes.
Answer:
Heavy water
11. By the reaction of A1 with concentrated ………………….. solution, Hydrogen is released.
Answer:
NaOH
12. Structure of hydrogen peroxide is like ………………….. .
Answer:
Open book
13. Mixture of CO + H2 is known as ………………….. .
Answer:
Water-gas
14. Volume ability of 2N H2O2 will be ………………….. .
Answer:
11.2 volume
15. Absorption of hydrogen by platinum is called ………………….. .
Answer:
adsorption.
![]()
3. Match the following:
| ‘A’ | ‘B’ |
| 1. Aqueous solution of copper sulphate is | (a) Deuterophosphine |
| 2. Aqueous solution of sodium carbonate is | (b) Oil gas |
| 3. Calcium phosphide react with heavy water | (c) H2O2 |
| 4. Distillation of kerosene oil form | (d) Acidic |
| 5. Which substance is used to wash statues to turn white from black | (e) Acidic |
| 6. Aqueous solution of ferric chloride is | (f) Basic |
| 7. Hydrogen peroxide H2O2 | (g) Moderator |
| 8. Heavy water D2O | (h) Rocket fuel |
| 9. Liquid H2 | (i) Insecticide |
Answer:
1. (d) Acidic
2. (f) Basic
3. (a) Deuterophosphine
4. (b) Oil gas
5. (c) H2O2
6. (e) Acidic
7. (i) Insecticide
8. (g) Moderator
9. (h) Rocket fuel.
4. Answer in one word/sentence:
1. In which compound oxidation state of hydrogen is negative?
Answer:
CaH2
2. Bleaching property of H2O2 is due to oxidation or reduction.
Answer:
H2O2 gives oxygen due to which it acts as a bleaching substance.
Thus, bleaching of H2O is due to oxidation.
H2O2 → H2O + [O]
3. Who discovered hydrogen?
Answer:
Henry Cavendish
4. Formula of heavy water is :
Answer:
D2O
5. What is used in the form of a moderator?
Answer:
Heavy water (D2O)
6. What is the mixture of CO and H2 gas known as?
Answer:
Water gas
7. H2O2 reduces Cl2 to which compound ?
Answer:
To HCl
8. Bleaching property of H2O2depend on :
Answer:
Due to nascent oxygen
9. How many isotopes of hydrogen are there?
Answer:
Three isotopes of hydrogen are: 1H1, 1H2,1H3
10. Which oxide reacts with dilute acid to form H2O2?
Answer:
Na2O2, BaC2
11. Ethanol vaporizes faster than water. Why?
Answer:
Due to weak hydrogen bond
12. Which type of elements form interstitial hydrides?
Answer:
Elements of d and f block
13. What is the use of intersititial hydride?
Answer:
In storage of H2 and catalysing hydrogenation reactions.
![]()
5. State true or false:
1. Pure H2O2 is a weak acid.
Answer:
True
2. Temporary hardness is removed by boiling water.
Answer:
True
3. Acetylene is formed by the action of CaH2 with water.
Answer:
False
4. Boiling point of hard water is less than that of soft water.
Answer:
False
5. 2D is an isomer of 1H.
Answer:
False
6. H2O2 cannot be reducing agent.
Answer:
False
7. NH3 and PH3 are saline hydrides.
Answer:
False
8. D2O is more polar than H2O.
Answer:
False
9. H2O is an example of ionic hydride.
Answer:
False.