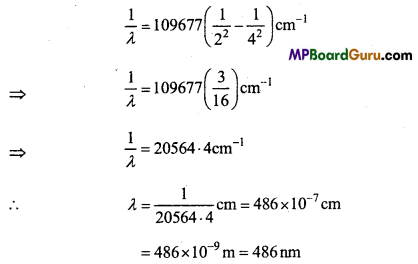
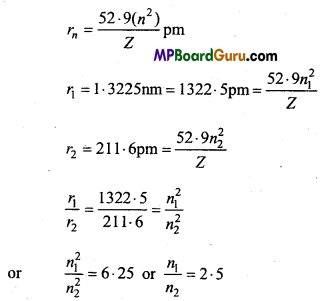
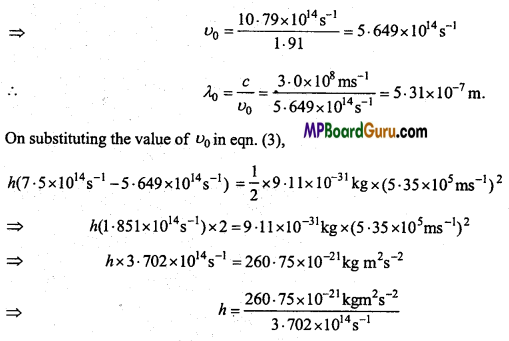
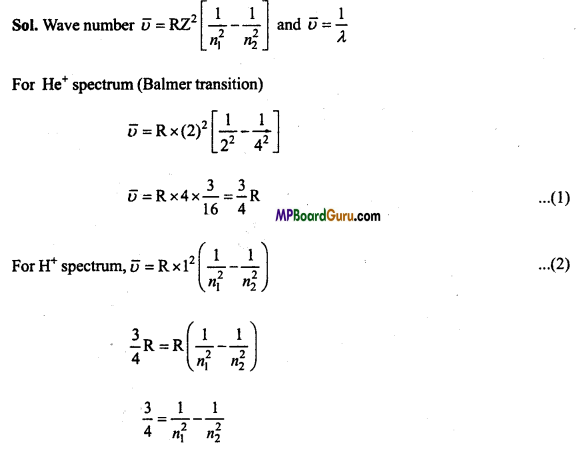
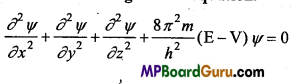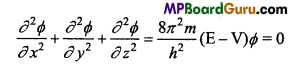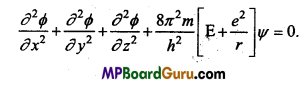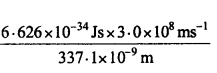These MP Board Class 11th Biology Notes for Chapter 17 Breathing and Exchange of Gases help students to get a brief overview of all the concepts.
MP Board Class 11th Biology Notes Chapter 17 Breathing and Exchange of Gases
→ Process if oxidation of food inside the cells of the living organisms in presence of specific enzymes to release energy is called as Respiration.
→ Respiration which occurs in presence of oxygen is called as aerobic respiration and which occurs in absence of oxygen is called as anaerobic respiration.
→ In modem birds, the lungs are highly modified by the presence of air sacs which facilitate the circulation of air through the lungs.
![]()
→ Inflammation of the pleural membrane called pleurisy causes friction during breathing which is quite painful.
→ In males, the larynx increases considerably in size and becomes much larger than that of the females. The enlarged vocal cords are responsible for lower pitch of the masculine voice.
→ Normally, trachea bifurcates into two bronchi but in snakes, there is only one bronchus while in certain ruminants, pigs and whales there are three bronchi.
→ The human lungs have about 750,000,000 alveoli covering the total area of 100 sq. meter which is 100 times than the surface area of the skin.
→ All tissues are not equally affected by the shortage of oxygen. The more active tissues are more affected. Skeletal muscles, heart and brain are much more affected than skin, intestine and bones.
→ Turtles can respire inside water due to the presence of air sacs in their body.
→ O2 is transported by haemoglobin in our body.
![]()
→ R.B.Cs. do not respire aerobically because of the absence of mitochondria in them.
→ Tfre rate of human breathing is 16-20 per minute at rest.
→ Hyperpnoea is a condition, when breathing rate increases above normal.
→ Hypopnoea is a condition, when breathing rate decreases below normal.
→ In some turtles, cloaca acts as respiratory organ.
→ Respiration is a catabolic process.
→ Gills are the respiratory organs of aquatic animals like fish.
→ Spiracles are small apertures in abdominal segments of insects for gaseous exchange.
→ Asthma : Narrowing of bronchi due to spasm in bronchial muscles. It is an allergic response.
![]()
→ Haemocyanin : Copper containing blue coloured pigment found in blood plasma of molluscs.

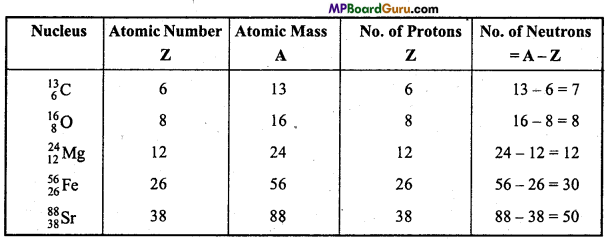


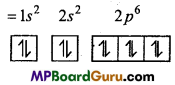
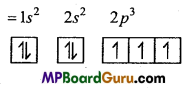
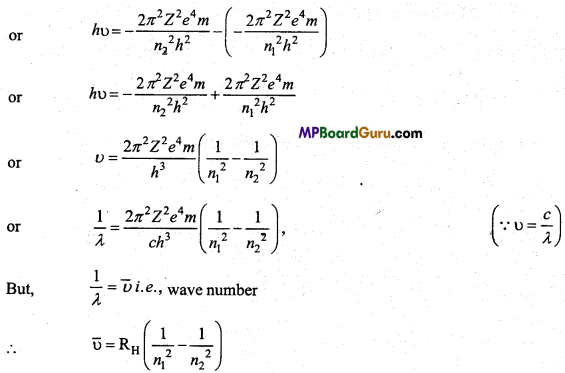
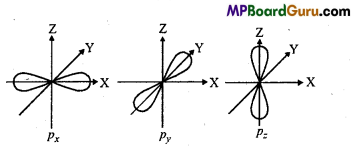
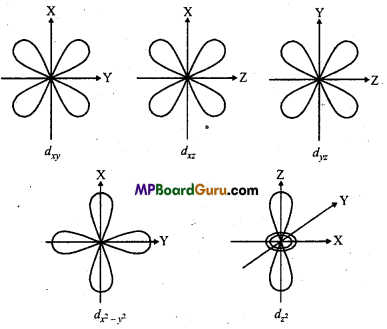
 n = 3, l = 1,m = -1,s = +\(\frac{1}{2}\)
n = 3, l = 1,m = -1,s = +\(\frac{1}{2}\)