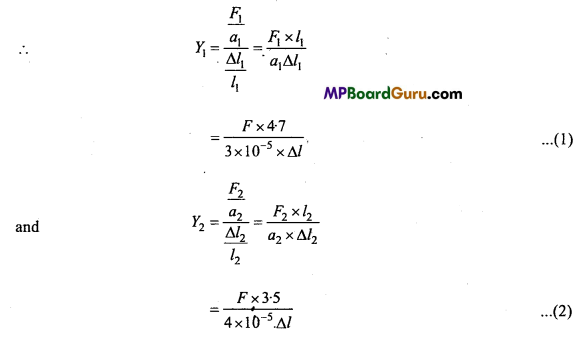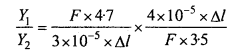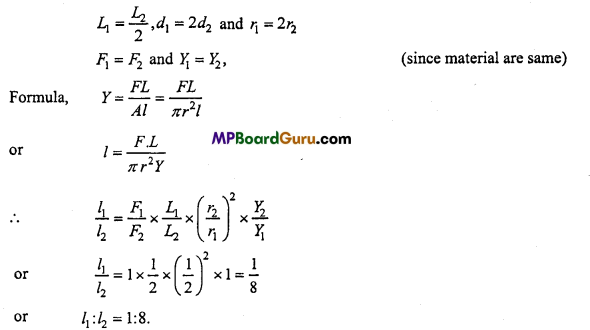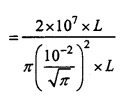These MP Board Class 11th Biology Notes for Chapter 7 Structural Organisation in Animals help students to get a brief overview of all the concepts.
MP Board Class 11th Biology Notes Chapter 7 Structural Organisation in Animals
→ The term morphology refers to the study of external structure. Earthworm is an annelid that has segmented body and looks like a snake. It has about 100 to 120 segments with metameric segmentation.
→ The mouth is situated in the first segment. A single median female genital pore opens on the ventral side of the 14th segment. A pair of male genital pore is present on the ventral side of the 18th segment. A prominent band encircles 14th, 15th and 16th segments. This is known as clitellum.
→ Cockroach has the characteristic jointed legs and is nocturnal in its habit. The body is segmented externally and divisible into a number of segments under head, thorax and abdomen.
![]()
→ The head is somewhat pear shaped which ariculated with the thorax by flexible neck. It bears compound eyes, antennae, mouth parts. Thorax is three segmented bearing jointed appendages. There are ten segments in abdomen.
→ Alimentary canal is well developed and is divisible into foregut, midgut and hind gut, Malpighian tubules are present at the junction of fore and mid gut and help in excretion. Respiration occurs by trachea. The blood vascular system is of open type. Fertilization is internal.
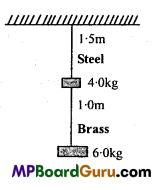
→ Frog is an amphibian that lives in water or on land near water. Its skin is soft, smooth and moist. The male frog bears copulatory pads and well developed vocal sac on the ventral surface of body cavity.
→ Body is divisible into head and trunk. It can respire through skin in water and through lungs on land. Circulatory system is closed with single circulation. The male reproductive organ is a pair of testes. The female reproductive organ is a pair of ovaries.
→ Rat is a mammal and its body is divided into head, neck, trunk and tail. Integument is made up of epidermis, dermis and their derivatives. Closed and double circulatory system is present.
→ Heart is four chambered. Six to eight young ones are produced in a litter.
![]()
→ Glucose is the main nutrient in the blood.
→ The total volume of E.C.F. (extracellular fluid) in adult human being is about 15 litres i.e., about 45% of the total body water.
→ Spongy bone occurs in the deeper central parts of bones. It has no concentric organization like haversian system. It consists of a network of many fine irregular bony plates called trabeculae.
→ In whale thick layer of adipose tissue is called blubber.
→ Ligament is a strong band of elastin connecting the two bones at a joint and holds them in position preventing dislocation.
→ Tendon is a collagenous connective tissue which connects a muscle to a bone or cartilage.
→ The average lifespan of R.B.Cs. in human beings is 120 days.
→ Increase in the number of R.B.Cs. in our body is called as polycythemia.
→ The membrane which covers the muscle fibres is called sarcolemma.
→ Largest muscle of human body is buttock muscles (Gluteus maximus).
→ Smallest muscle of human body is stapedius. .
→ Riger mortis : The rigidity and non-elasticity of muscles after death. It is first seen in jaw muscles.
→ Myology : Study of muscles.
![]()
→ The process of the formation of blood corpuscles is known as haemopoiesis.
→ Decomposition of blood cells is called as haemolysis.
→ Collagen and elastin is the fibrous protein found in connective tissue.
→ The amount of haemoglobin is 15 gm per 100 ml of blood.
→ Abnormal decrease in the number of R.B.Cs. in the blood is called as anaemia.
→ Excessive stretching of ligaments is called sprain.
→ Haversian systems are absent in spongy bones of mammals.
→ Myoglobin protein is called as muscle haemoglobin.
→ Platelets occur in mammals only.
![]()
→ Muscular tissues are formed from the mesoderm layer of embryo.
→ Striated muscles contain acetylcholine which stimulates their contraction.
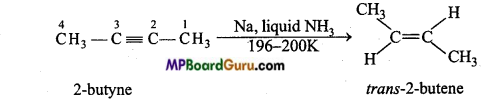
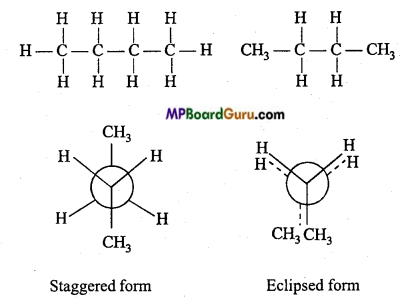
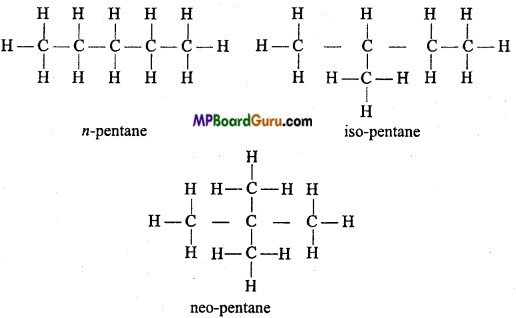
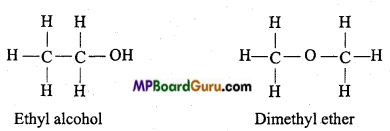
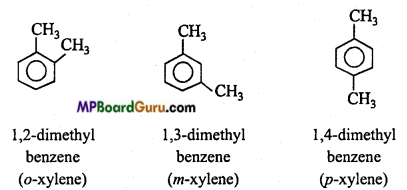
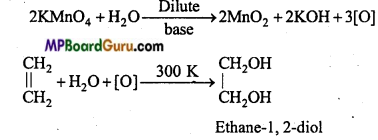
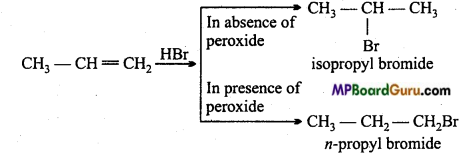
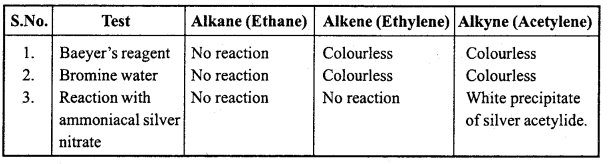



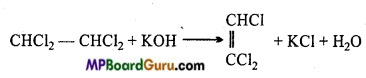
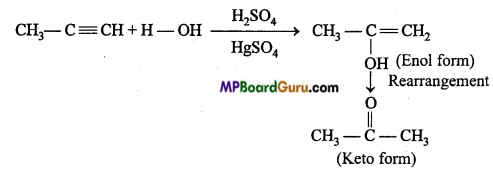
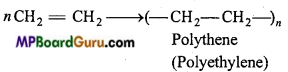
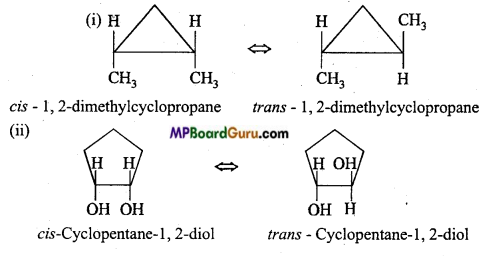
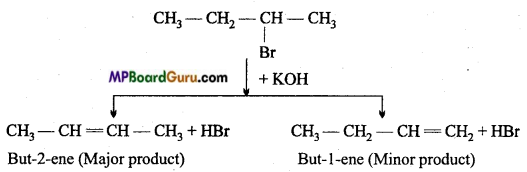
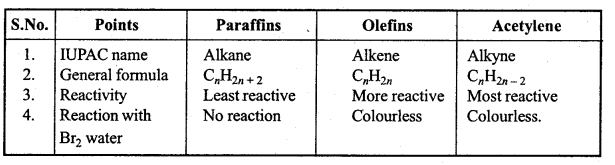
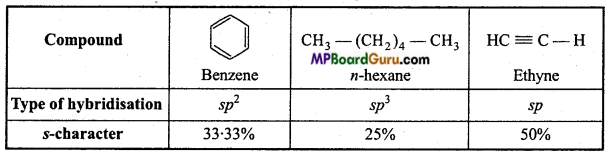
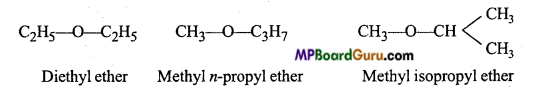


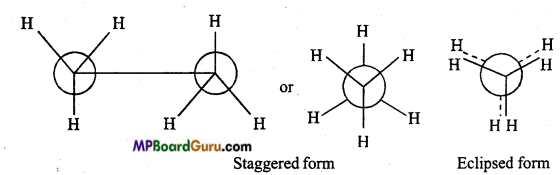
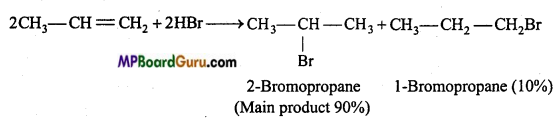
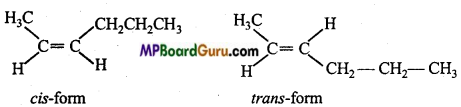
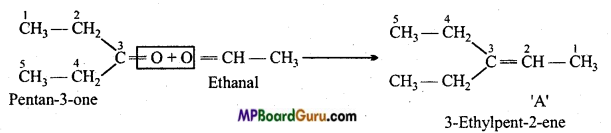
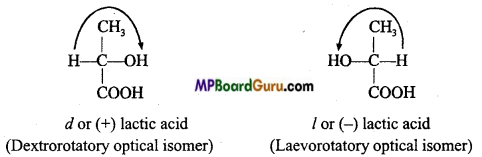
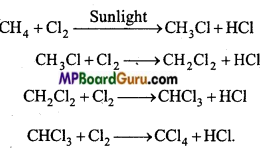
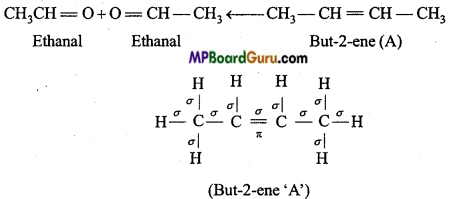
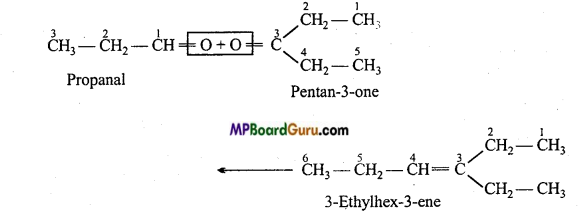

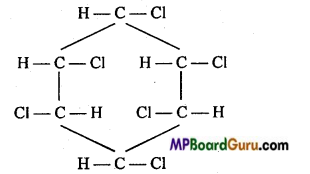
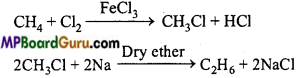
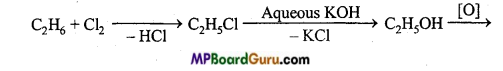
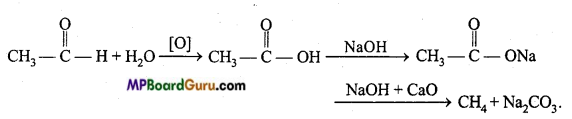
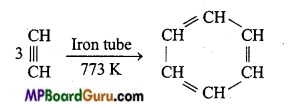
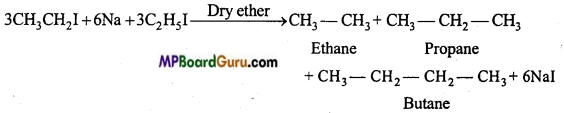

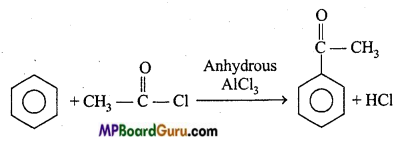

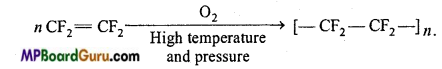
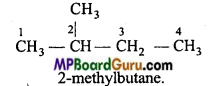
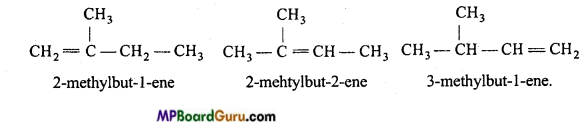
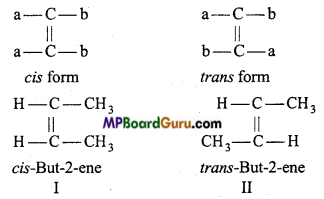
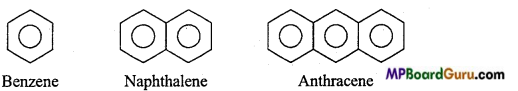

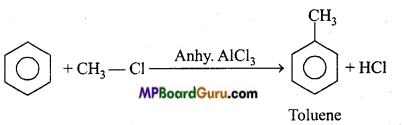
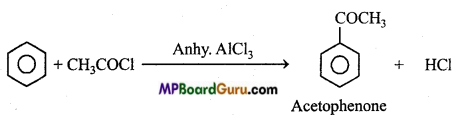

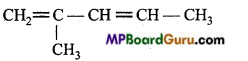
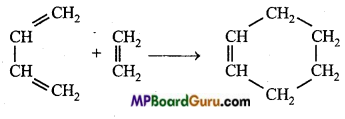

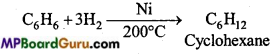
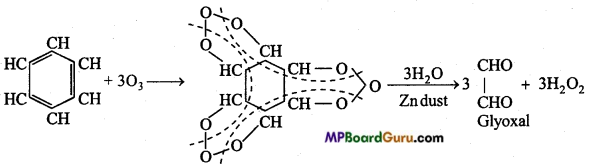
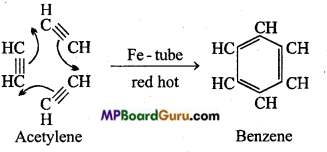

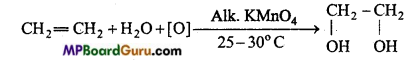
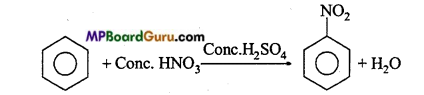

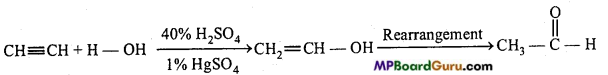
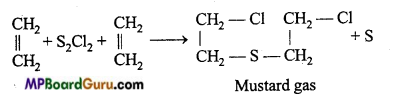
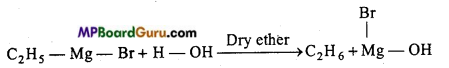
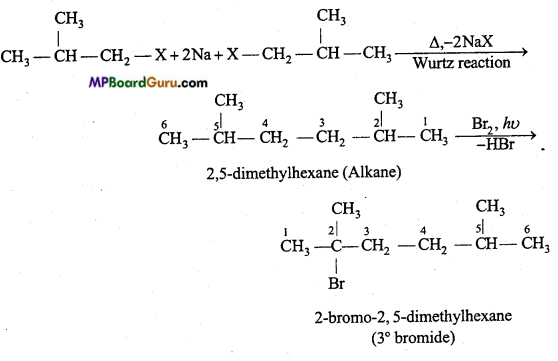
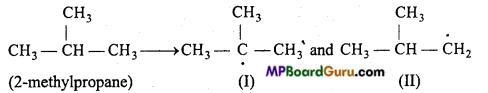
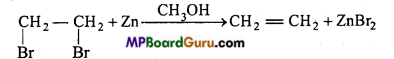
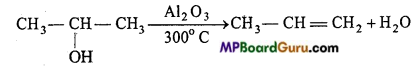
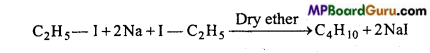

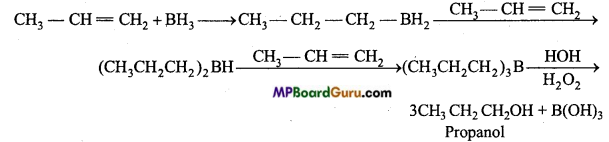
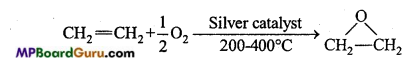


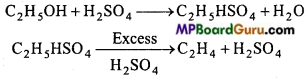
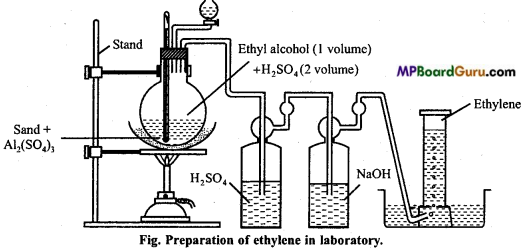
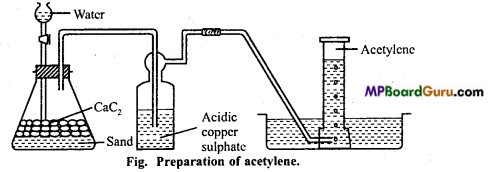
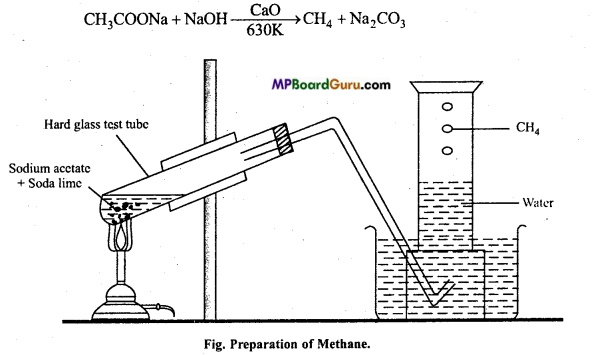

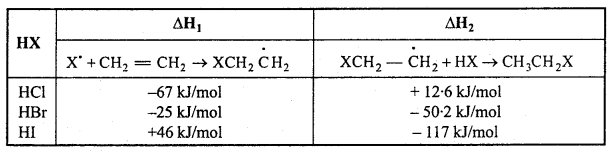
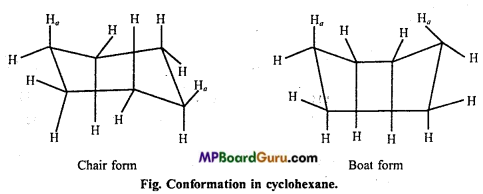
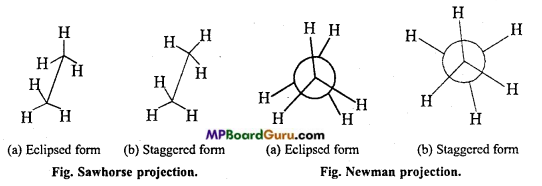
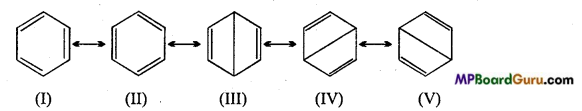
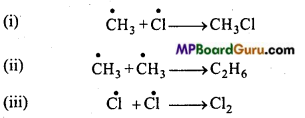
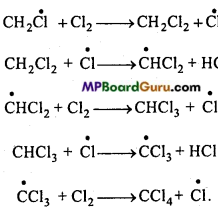
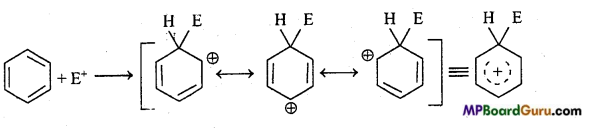
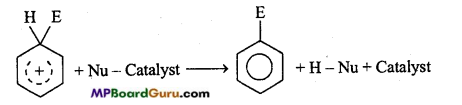

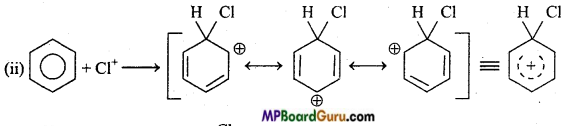
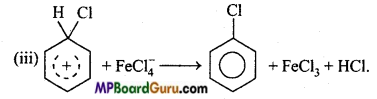
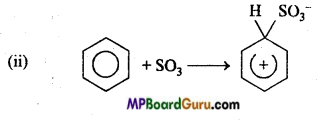
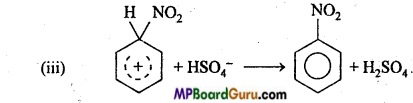
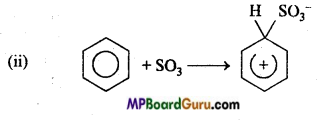
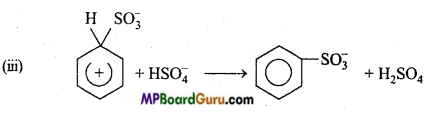
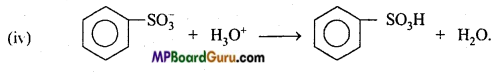
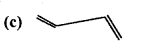

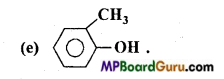
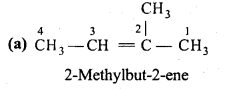
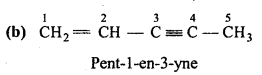
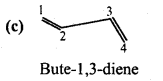
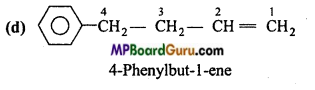
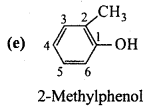
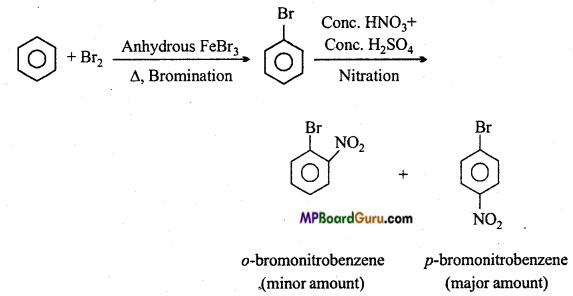
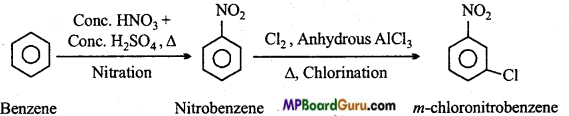
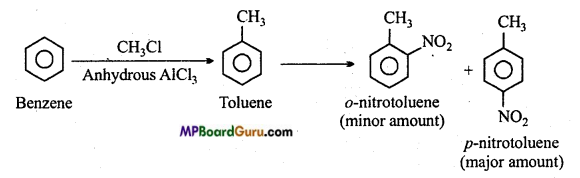
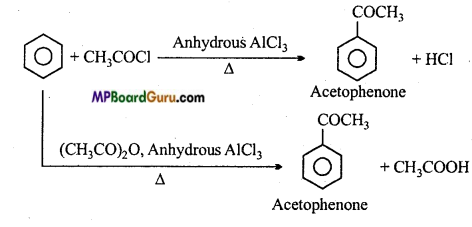
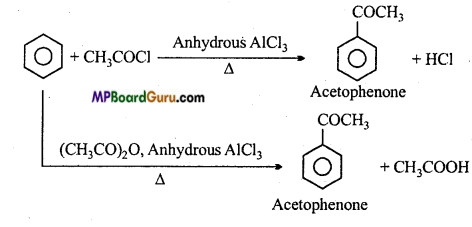
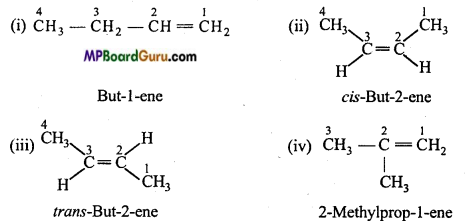

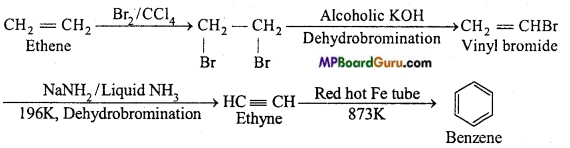
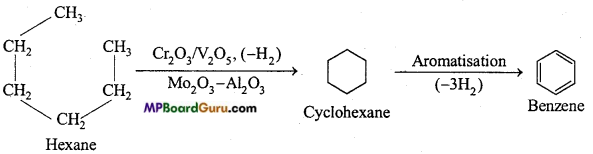

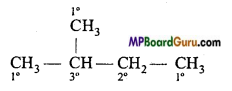

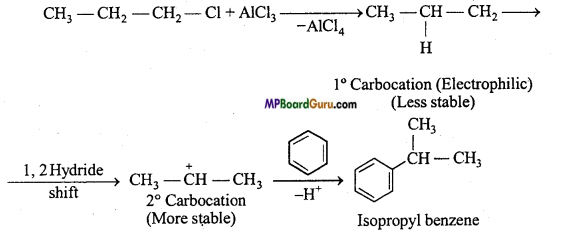
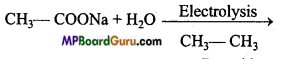
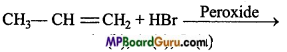
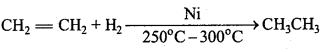 is
is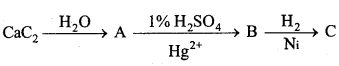 write the name of the products formed in the reaction.
write the name of the products formed in the reaction.