Students get through the MP Board Class 11th Chemistry Important Questions Chapter 6 Thermodynamics which are most likely to be asked in the exam.
MP Board Class 11th Chapter 6 Thermodynamics
Thermodynamics Class 11 Important Questions Very Short Answer Type
Question 1.
Tell the meaning of the word Chemical Energetics.
Answer:
In Chemical Energetics, energy changes associated in chemical reactions is studied.
Question 2.
Value of Kp for reaction N2O4(g) ⇌ 2NO2(g) at 298 K is 0.98. State whether the process will be spontaneous or not.
Solution:
ΔrG° = -2303 RT log P
∴ Kp = 0.98 i.e. Kp < 1
∵ Δr G° is positive. Thus the process will be non-spontaneous.
Question 3.
Extensive property depend upon the amount of matter but intensive property is independent of the amount of matter. State whether the following properties are extensive or intensive? Mass, Internal energy, Pressure, Heat capacity, Molar heat Capacity, Density, Mole fraction, Specific heat, Temperature and Molarity.
Answer:
Extensive property: Mass, Internal energy, heat capacity,
Intensive property: Pressure, molar heat capacity, density, mole fraction, specific heat, temperature and molarity.
Question 4.
Heat capacity (P) is an Extensive property whereas specific heat (c) is an intensive property. For 1 mole water. What is the relation between Cr and C?
Solution:
Work done, W = -PExternal (V2 – V1)
When PExternal = 0
Thus, W = -0(5-1) = 0
For an Isothermal Expansion,
ΔU = 0
Thus, ΔT = 0.
![]()
Question 5.
What is a system?
Answer:
System: A specified portion of the universe which is selected for experimental or theoretical investigations is called the system. In the system, the effects of certain properties such as pressure, temperature, etc. are observed.
A system is said to be homogeneous if it consists of only one phase, e.g. mixture of gas On the other hand, it is heterogeneous if it consists of more than one phases, e.g., Mixture of water and oil.
Question 6.
What is a Thermodynamic process? It is of how many types?
Answer:
System which cause change in a thermodynamic state is called Thermodynamic process. ,
Types of Thermodynamic process :
- Isothermal process,
- Adiabatic process,
- Isobaric process,
- Ischoric process,
- Reversible process,
- Irreversible process,
- Cyclic process.
Question 7.
What is Exothermic reaction? Explain with example. Or, For Exothermic reaction, sign of ΔH is negative. Why?
Answer:
Exothermic reactions: The reactions in which heat is emitted are called exothermic reactions. In these reactions total heat of products is less than that of reactants.
The heat emitted or evolved during the reaction thus enthalpy of such reactions, is negative.
ΔH = HProduct – HReactants
∵ HProduct < HReactants
∴ ΔH = -ve .
Example : 2NO,sub>(g)→ N2(g) + O2(g);
ΔH =-180.5 kJ
Question 8.
Explain Intensive and Extensive properties.
Answer:
Intensive properties: The properties of the system which are independent of the amount of matter present in it, are called intensive properties.
Example: Temperature, viscosity, surface tension, refractive index, specific heat, density, etc.
Extensive properties: The properties of the system which depend upon the amount of matter present in it, are called extensive properties.
Example: Mass, volume, energy, etc.
Question 9.
What is the zeroth law of Thermodynamics?
Answer:
This law is also known as law of thermal equilibrium. According to A this law. “If two bodies are separately in thermal equilibrium of a third body, then they are also in thermal equilibrium with each other”.

Question 10.
Define Heat of neutralization.
Answer:
Heat of neutralization: Heat of neutralization is the change in enthalpy of system when one gram equivalent of any acid neutralize completely with one gram equivalent of base.
For example:
HCl(aq)+ NaOH(aq) → NaCl(aq) + H20(l);
ΔH = -57.1 kJ.
![]()
Question 11.
Value of heat of neutralization of weak acid and strong base is less than value of heat of neutralisation of strong acid and strong base. Why?
Answer:
Value of heat of neutralisation of weak acid and strong base is less because some part of heat evolved is utilized in complete dissociation of weak acid or base. Thus, as a result, in the neutralization of weak acid and base by strong base or strong acid value of heat obtained is less than the value of heat obtained by the neutralization of strong acid and strong base.
HCl + NH4OH → NH4Cl+H2O;
ΔH = 12.4kcal
Strong acid weak base.
Question 12.
What is Bond dissociation energy or Bond enthalpy?
Answer:
Energy is released during the formation of a chemical bond. Thus, energy is required for the breaking of a bond. Energy required for breaking a bond is called enthalpy. Thus, it is enthalpy change required to break up a gaseous molecule into atoms.
HCl(g) → H(g) + Cl(g)
ΔH = 431 KJ/mol
Question 13.
What is the first law of thermodynamics?
Answer:
First law of thermodynamics is the law of conservation of energy. The common statement of this law is :
“Energy can neither be created nor be destroyed but it can be converted from one form to another form.”
Let internal energy of the system is E1 and q calorie heat is supplied to the system. E2 is the energy of the final stage and work done is W. Therefore,
E2 – E1 = q + W
or ΔE = q + W.
Question 14.
What is meant by Entropy? Explain the change in entropy with an example.
Answer:
Entropy is a measure of degree of disorder or randomness of the system. Greater the disorder of the system higher is its entropy. For a given substance the crystalline state represents the state of lowest entropy, gaseous state represents the state of highest entropy and the liquid is the intermediate between the two. Absolute value of entropy cannot be determined but entropy change AS can be calculated as
ΔS = Sfinal – Sinitial
= S2-S1
= \(\frac{q_{\text {rev }}}{T}\).
Where qrev means that heat is given to the system in a reversible from unit of ΔS is JK-1.
Question 15.
Among water vapour, water and ice whose entropy is more and why?
Answer:
Entropy is the measure of irregularity. In solid state, molecules are completely arranged, therefore its entropy is minimum and gas molecules move in indefinite form in all directions, therefore its entropy is maximum.
S(ice) < S(water) < S(water vapour).
Question 16.
Among NaCl, H2O and NH3 whose entropy is maximum and why?
Answer:
Entropy is a measure of disorder of a substance. Among solids, liquids and gases, maximum disordemess is found in gases. In the above example, NaCl is solid, H2O is liquid and NH3 is a gas. So, entropy of NH3 will be maximum.
Question 17.
Prove that: PAV = ΔnRT.
Answer:
By Ideal gas equation :
PV = nRT
If in initial state, volume of gas is V1 and number of moles is n1 then,
PV1 =n1RT ………………… (i)
If in final state, volume of gas is V2 and number of moles is n2, then,
PV2 =n2RT ………………….. (ii)
By eqn. (i) and (ii),
P(V2 – V1) =(n2 – n1)RT
PΔY = Δ nRT.
![]()
Question 18.
What is the relation between ΔH and ΔU?
Answer:
If enthalpy of a system is H and internal energy is U then the relation between enthalpy and internal energy is as follows :
H=U+PV
For enthalpy change ΔH = ΔU + PΔV
We know that PΔV = ΔnRT On substituting the value,
ΔH = ΔU +ΔnRT
Question 19.
What is meant by specific heat capacity?
Answer:
Heat required to rise the temperature of one gram of a substance by 1 degree is called specific heat capacity. It is represented by Cs.
Cs = \(\frac{\mathrm{C}}{\mathrm{m}}\)
Where, C = Heat capacity, Cs = Specific heat capacity, m = Mass of substance.
Its S.I. unit is joule per kelvin per gram.
Question 20.
What is meant by molar heat capacity?
Answer:
Heat required to increase the temperature of one mole of a substance by one degree is called molar heat capacity. It is represented by Cm.
Molar heat capacity Cm = \(\frac{\mathrm{C}}{n}\)
Where C = Heat capacity, q = Heat absorbed, ΔT = Increase in temperature, n = Number of moles.
Its S.I. unit is joule per kelvin per mole.
Question 21.
What is Hess’s law of constant heat summation?
Answer:
In 1840, G. H. Hess gave an important law of constant heat summation according to which, “The enthalpy change in a particular reaction is always constant and does not depend on the path in which reaction takes place”.
Or
“The enthalpy change in a physical or chemical process is the same whether the process is carried out in one or in several steps.”
Question 22.
What is an adiabatic process? Explain extensive and intensive properties.
Answer:
Adiabatic process: It is a process in which the system does not exchange heat with its surroundings, i.e., no heat enters or leaves the system. Adiabatic process occurs in isolated systems. For such type of process
dq = 0.
Question 23.
What is meant by standard enthalpy of formation?
Answer:
Standard enthalpy of formation: The heat change taking place during the formation of 1 mole of a compound from its constituent elements under standard conditions of 1 atmospheric pressure and 298 K temperature is called standard enthalpy of formation. It is represented by ΔH0f or ΔfH° .
Question 24.
What is enthalpy of solution? Explain with example.
Answer:
Heat change when 1 mole of a substance completely dissolves in excess of solvent is called enthalpy of solution. Excess of solvent means after the formation of solution there should by no heat change on adding solvent in it.
Example: KCl(s) + aq → KCl(aq); ΔH = +18.6kJ
Question 25.
Define enthalpy of hydration.
Answer:
Enthalpy of hydration (ΔHydH) : It is defined as the amount of heat evolved or absorbed when one mole of anhydrous salt combines with the required number of moles of water to form hydrated salt. For example, the heat of hydration of anhydrous copper sulphate is -78.2 kJ mol-1.
CUSO4(s)+5H2O(l) → CuSO4.5H2O(s); ΔHydH = -78.2 kJ.
![]()
Question 26.
What is meant by enthaply of fusion?
Answer:
When one mole solid changes into liquid at its melting point and one atmospheric pressure, the enthalpy change in this process is called enthalpy of fusion.
For example, the melting point of ice, 6.01 kJ heat is required to convert one-mole ice into one-mole water.
H2O(s) → H2O(l);
ΔH = +6.01 kJ
Heat of fusion of ice at 273 K = 6.01 kJ.
Question 27.
For an isolated system ΔU = 0, what will be ΔS for it ?
Answer:
For an isolated system, ΔU = 0 and for a spontaneous process, total change in entropy should be positive. .
For example: In a closed container which is isolated from the surroundings, two gases A and B are diffused, both the gases are separated by a mobile divider. When the divider is removed, then the gases start diffusing mutually and the system becomes more disordered.
For this system, ΔS > 0 and ΔU = 0.
Again ΔS = \(\frac{q_{\text {rev }}}{\mathrm{T}}\) = \(\frac{\Delta \mathrm{H}}{\mathrm{T}}\) = \(\frac{\Delta \mathrm{U}+\mathrm{P} \Delta \mathrm{V}}{\mathrm{T}}\) =\(\frac{\mathrm{P} \Delta \mathrm{V}}{\mathrm{T}} \) (∵ΔU =0)
Thus, TΔS or ΔS >0.
Thermodynamics Class 11 Important Questions Short Answer Type
Question 1.
What is the first law of thermodynamics? Write its mathematical form.
Answer:
First law of thermodynamics is the law of conservation of energy. The common statement of this law is :
“Energy can neither be created nor be destroyed but it can be converted from one form to another form. ”
Let internal energy of the system is U1 and q calorie heat is supplied to the system. U2 is the energy of the final stage and work done is W, Therefore,
U2 = U1+q + W
or
U2 – U1 = q+ W .
or ΔU = q+ W,
(∵ U2 – U1 = ΔU)
Thus, change in internal energy = Heat given to the system + Work done on the system.
Question 2.
First law of Thermodynamics is sometimes expressed as ΔU = q- W and sometimes as ΔU = q + W. Why?
Answer:
If work is done by the system on the surroundings, then some energies of the system is used up in doing work due to which internal energy of the system decreases and work done by the the system, is negative. In this condition, first law of thermodynamics is expressed as ΔE = g-W.
Alternatively, if work is done on the system by the surroundings then work done is positive because internal energy of the system increases and in this condition by the first law of thermodynamics is expressed as ΔU = q + W.
Question 3.
Differentiate between Reversible and Irresversible Process.
Answer:
Differences between Reversible and Irresversible Process
| Reversible Process | Irreversible Process |
| 1. These process are carried out infinitesimally slowly. In it the difference between driving force and opposing force is very small. | 1. These process are carried out rapidly. In it the difference between driving force and opposing force is sufficiently large. |
| 2. These are mostly theoritical. | 2. These are mostly natural and spontaneous. |
| 3. Large time is required for their completion. | 3. A definite time is required for their completion. |
| 4. Work done in these process is maximum. | 4. Work dope in these process is not maximum. |
| 5. At any stage during the process equilibrium is not disturbed. | 5. Equilibrium may exist only after the completion of the process. |
Question 4.
On the basis of the following reactions comment on thermodynamic stability of NO(g):

Answer:
NO(g) is unstable because manufacture of NO is an endothermic process (i.e., heat is absorbed) but NO2 is stable because its formation is an exothermic process (i.e., heat is evolved). Thus, unstable NO(g) changes to stable NO2(g).
Question 5.
At equilibrium, which quantity ΔrG or ΔrG° will have zero value ?
Answer:
ΔrG = ΔrG°+ RTlnK
At equilibrium, 0 = ΔrG°+ RTlnK
or ΔrG° =-RTlnK
or ΔrG°= 0, (When K= 1)
For other values of K,
value of ΔrG° will be zero.
Question 6.
Determine the internal energy change of an isolated system at constant volume.
Answer:
Energy of an isolated system cannot be transformed into heat or in the form of work. Thus, by the first law of thermodynamics :
ΔU = q + W
ΔU = 0 + 0=0.
![]()
Question 7.
What is heat capacity? Establish the following expression : Cp – Cv = R.
Answer:
Heat Capacity: The amount of heat required to increase the temperature of a system by 1°C is called heat capacity. Its 5.1. unit is joule per kelvin.
Heat capacity at constant volume,
Cv = \(=\left(\frac{\Delta \mathrm{U}}{\Delta \mathrm{T}}\right)_{\mathrm{V}}\)
or ΔU = CvΔT = qv …….. (i)
Similarly heat capacity at constant pressure,
Cv = \(\left(\frac{\Delta \mathrm{H}}{\Delta \mathrm{T}}\right)_{\mathrm{P}}\)
or ΔH = CPΔT = qP ………. (ii)
For 1 mole of an ideal gas,
ΔH = ΔU + Δ(PV)
or ΔH = ΔU + Δ(RT), (∵ PV =RT)
or ΔH = ΔU + RΔT
Substituting the values of ΔH and ΔU from eqn. (i) and (ii),
CpΔT = CVΔT + RΔT
On dividing by ΔT,
Cp = Cv + R
Cp -Cv=R
Question 8.
(a) Explain heat of combustion with example.
(b) Write four applications of heat of combustion.
Answer:
(a) Heat of combustion: Heat of combustion of any substance is the quantity of heat liberated when one mole of that substance completely reacts with excess of air or oxygen.
Example:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l); ΔH= -890.3 kJ
C(s)+O2(g) → CO2(g): ΔH=—593.5kJ
C6H6(l) + 7\(\frac{1}{2}\)O2(g) → 6CO2(g) + 3H2O(l); ΔH = -3268 KJ
(b) Applications of enthalpy of combustion:
- In determination of calonfic value of fuel.
- In determination of heat of formation of compounds.
- In determination of structure of compounds.
- In calculation of calorie value of food.
Question 9.
Expansion of a gas in vacuum ¡s known as free expansion. If 1 L of an ideal gas by isothermal expansion expands to 5 L, then determine work and change in internal energy.
solution :
Work done, W = -PExternal (V2 – V1)
When PExternal = 0
Thus, W = – 0(5 – 1) = 0
For isothermal expansion,
ΔT = 0
Thus, ΔU = 0.
Question 10.
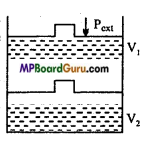
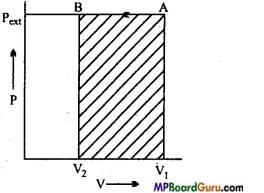
A gas enclosed in a cylinder as ¡n figure is compressed by a definite external pressure P, then how much work will be done on the gas? Explain by graph.
Answer:
Suppose, initially total volume of the gas is V1 and pressure of the cylinder gas is P. On applying external pressure Pext in single step, it compresses and the final volume becomes V2.
Thus, change in volume V = (V2 – V1)
If due to motion of the piston, W work is done on the Pext system, then work done
W =-Pext ( ΔV)
W=- Pext (V2 – V1)
or
W=Pext (V1 -V2), (∵ V2 < V1)
It can be obtained by P – V graph in the figure. Work done is equal to the shaded portion ABV2V1. Positive work expresses that work is done on the system.
Question 11.
Explain enthalpy of vaporization and formation.
Answer:
Enthalpy of vaporization: Enthalpy change in vaporization of 1-mole liquid at its boiling point and I atm pressure, is called enthalpy of vaporization.
For example: H2O(l) → H2O(g); ΔH =+ 40.7 kJ
∴ Heat of vaporization of water at I atm pressure = +40.7 KJ.
Enthapy of formation: The enthalpy of formation is the enthalpy change in the process in which one mole of compound is formed from its elements. It is denoted by ΔHf. Enthalpy of formation of carbon dioxide is given below:
C(s) + O2(g) → CO2(g); ΔH = ΔHf = -393.5 kJ
Question 12.
Explain enthalpy of fusion and sublimation with example.
Answer:
Enthalpy of fusion: When one mole solid changes into liquid at its melting point and one atmospheric pressure, the enthalpy change in this process is called enthalpy of fusion.
For example, the melting point of ice, 6.01 kJ heat is required to convert one-mole ice into one-mole water.
H2O(s) → H2O(l);
ΔH = +6.01 kJ
Heat of fusion of ice at 273 K = 6.01 kJ.
![]()
Enthalpy of sublimation: The enthalpy change when one mole of a solid is directly converted into gaseous state without changing into liquid state at a temperature below its melting point is called enthalpy of sublimation.
I2(s) ⇌ double arow I2(g)
ΔH = +62.4 kJ.
Question 13.
How does entropy change in the process of vaporization?
Answer:
Entropy of vaporization: The entropy change when one mole liquid at its boiling point and one atmospheric pressure changes in vapour state is called Entropy of vaporization.
Suppose at constant pressure one mole liquid at its boiling point. Tb, change from liquid to vapour state in a reversible form. If molar heat of vaporization of this substance is ΔHvap then entropy change ΔSvap can be expressed as follows :
ΔSvap = \(\frac{\Delta \mathrm{H}_{\text {fus }}}{\mathrm{T}_{b}}\)
Since, both ΔHfus and ΔHvapare positive, therefore in both the process, vaporization and fusion there is increase in entropy.
Question 14.
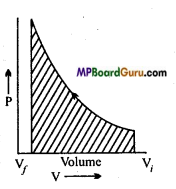
How will you calculate the work done in compression of an ideal gas when change in pressure is done in infinite p steps?
Answer:
When change in pressure is done in infinite steps then it is a reversible process. Thus, work done is shown by the shaded portion.
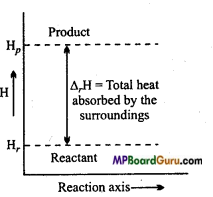
Question 15.
For a reaction, figure of enthalpy is represented. Is it possible to decide the spontaneity of the reaction by the given figure?
Answer:
No, enthalpy is one of the factor to decide the spontaneity of a reaction but it is not the only factor. For this, other factors like ΔG, ΔS, T, are also to be considered.
Question 16.
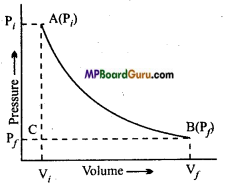
By reversible and isothermal process of an ideal gas changes in state occur from (Pi Vf) to (Pf, Vf). In this expansion represent the work done in graphical form. By the help of PV graph, compare the work done against constant external pressure with the above work?
Answer:
- Reversible work is represented by total area ABC and BC ViVf.
- Work done by constant pressure Pyis represented by area BC ViVf.
Thus, Work (i) > Work (ii).
Question 17.
What is internal energy?
Answer:
Each substance possesses a definite amount of energy which depends upon its chemical nature, temperature, pressure, volume, etc. Absolute value of this energy is called internal energy or intrinsic energy.
This energy is represented by E or U. Internal energy is the sum of different energies present in substance. Like :
- Translational energy of molecules (Et).
- Rotational energy of molecules (Er).
- Vibrational energy of molecules (Ev).
- Electronic energy (Ee).
- Nuclear energy (En).
- Energy due to molecular interactions (Ei).
So, E = Et+Er + Ev+ Ee + En+Ei.
Question 18.
What factors affect the enthalpy of reaction?
Answer:
Enthalpy of a reaction depends on the following factors :
1. Physical states of reactants and products: Physical states of reactants and products affect the enthalpy because latent heat is also included in it.
H2(g)+ \(\frac{1}{2}\)O2(g) → H2O(l) ΔH = -286kJ
H2(g) +\(\frac{1}{2}\)O2(g) →H2O(g) ΔH = -249 kJ
2. Amount of reactant: Enthalpy of a reaction also depends on the amount of reactant.
Like: 1 mole H2 reacts with \(\frac{1}{2}\) mole O2 to produce 286 kJ of heat. In 2 moles this quantity becomes double.
H2(g) + \(\frac{1}{2}\)O2(g) → H2O(l) , ΔH = -286 kJ
2H2(g) + O2(g)→ H2O(l), ΔH = -572 KJ
3. Allotropic transformation of reactants:Enthalpy of different allotropes of the same substance is different.
CGraphite + O2(g) → CO2(g); ΔH = -393.5 kJ
CDiamond + O2(g) → CO2(g); ΔH = -395.4kJ
4. Temperature: Enthalpy of a reaction also depends on temperature.
At 25°C : H2(g) + Cl2(g) → 2HCl(g); ΔH = -184.4 kJ
At 75°C : H2(g) + Cl2(g) → 2HCl(g); ΔH = +184.4 kJ
Question 19.
Prove that at constant volume qv = ΔU.
Answer:
When a reaction is performed at constant volume, then no work is done by the system.
Thus, W = 0
∴ ΔU = q + W
= q + 0
ΔU = q
Thus, the heat absorbed at constant volume is used to increase the internal energy of the system.
Thus, ΔU = q + W
or ΔU = 9+PΔV, (∵ W = PΔV)
Since, the reaction takes place at constant volume, therefore ΔV = 0
On substituting ΔU = 0.
![]()
Question 20.
Write five applications of Hess’s law.
Answer:
The applications of Hess’s law of constant heat summation are as follows :
- Calculation of bond energy,
- Calculation of enthalpy of transition of allotropes,
- Calculation of enthalpy of formation,
- Calculation of enthalpy of combustion,
- Calculation of enthalpy of hydration.
Question 21.
What is meant by calorific value of a fuel? Explain with example.
Answer:
Heat produced by the combustion of one gram food or fuel in the form of calorie or joule is called calorific value of food or fuel.
C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ; Δ H = -2840 KJ
In this reaction heat produced by 1 mole or 180gm of glucose = 2840 kJ.
Thus, heat obtained by 1 gm glucose = \(\frac{2840}{180}\) = 15.78 kJ/gm
Thus, calorific value of glucose = 15.78 kJ/gm.
Thermodynamics Class 11 Important Questions Long Answer Type
Question 1.
Prove that ΔH =ΔU + PΔV Or, Explain the relation between ΔH and ΔU.
Answer:
According to first law of Thermodynamics,
q = ΔU + W .
or
q = ΔU + PΔV, (∵W = PΔY) …………. (i)
According to enthalpy,
q = ΔH ………………… (ii)
By eqn. (i) and (ii)
ΔH = ΔU + PΔV ……………………. (iii)
By ideal gas equation,
PV = nRT
Let volume of gas in initial state is V1 and No. of moles of gas is n1 then,
PV1 =n1RT …………………. (iv)
In final state, volume of gas is V2 and No. of moles of gas is n2 then,
PV2 =n2RT ……………. (V)
Substracting eqn. (iv) from eqn. (v),
P(V,sub>2-v1) = (n2 – n1)RT or PΔV = ΔnRT
Substituting the value of PΔV in eqn. (iii),
ΔH=ΔU+ΔnRT
Conditions :
- If No. of moles of reactant is more than the No. of moles of product then A n will be positive and value of Δ H will be more than Δ U. Thus,
ΔH=ΔU+ΔnRT - If No. of moles of reactant is more than No. of moles of product then Δn will be negative and ΔH will be less than ΔU. Thus, ‘
ΔH=ΔU- ΔnRT - If No. of moles of reactant is equal to the No. of moles of product then Δn = 0. In this condition ΔH = ΔU.
Question 2.
Prove that qP = ΔH.
Or, Prove that at constant pressure and temperature, heat of reaction is equal to enthalpy change of the system.
Answer:
If a process takes place in constant pressure then there is change in volume. If energy is absorbed by the system, then as a result internal energy increases from U1 to U2 and volume increases from V1 to V2, then according to the first law of Thermodynamics :
q = ΔU + W =ΔU + PΔV, (∵ W = PΔV)
or q = [U2 -U1] + P[V2 – V1], [U2 -U, = ΔU, V2 – V1 = ΔV] or q =[U2-U1] + [PV2-PV1]
or q = [U2 +PV2]-[U1 +PV1] . …………………. (i)
Internal energy, pressure and volume are state variable properties, therefore their sum will also be state variable, which is also known enthalpy and represented by H
U + PV = H
If in initial state, internal energy, volume and enthalpy are U1, V1 and H1 then,
U1 + PV1 = H1 …………………… (ii)
Similarly, in final state, internal energy, volume and enthalpy are U2, V2 and H2 then,
U2+PV2 = H2 ………………………. (ii)
Substituting these values from eqn. (ii) and in eqn. (i),
q = H2 -H1
qp = ΔH
(Where, qp = Heat absorbed by the system at constant pressure).
![]()
Question 3.
What is Hess’s law of constant heat summation? Explain with an example.
Answer:
Hess’s law: When one mole solid changes into liquid at its melting point and one atmospheric pressure, the enthalpy change in this process is called enthalpy of fusion.
For example, the melting point of ice, 6.01 kJ heat is required to convert one-mole ice into one-mole water.
H2O(s) → H2O(l);
ΔH = +6.01 kJ
Heat of fusion of ice at 273 K = 6.01 kJ.
This law is based on the law of conservation of energy.
Suppose that the conversion of substance A to substance Z takes place in a single step by first method and through several steps in second method. In single step :
A → Z + Q1
Where, Q, is the energy evolved.
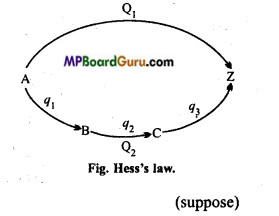
In case of several steps :
A → B +q1
B → C + q2
C → Z +q3
Total energy evolved in several steps = q1+q2+q3
= Q2 calories
According to Hess’s law,
Q1 =Q2
Suppose Hess’s law is incorrect and Q2 > Q1. In this stage if we convert A to Z by several steps and then Z directly to A, then heat equal to (Q2 – Q1) is produced. By repeating this cyclic process several times, an unlimited amount of heat (energy) may be produced in an isolated system. But this is against the law of conservation of energy.Practically also, Hess’s law is proved to be true.
Example: Carbon can be directly converted to CO2 by direct burning or in the other way be first converting it to carbon monoxide and then oxidizing it to carbon dioxide.
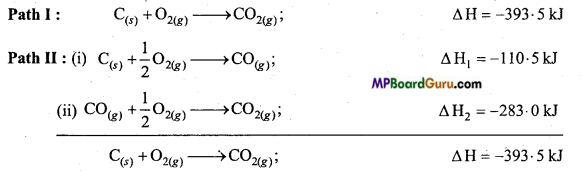
Which is equal to ΔH (enthalpy change in path I)
Hence, ΔH = ΔH, + ΔH2.
Question 4.
Explain the determination of internal energy change by bomb calorimeter under the following heads :
(i) Labelled diagram of the apparatus,
(ii) Explanation of the process,
(iii) Calculations.
Or, Draw a labelled diagram of bomb calorimeter. How can we find internal energy by it ? .
Answer:
Experimental determination of change in internal energy : The change in internal energy in a chemical reaction is determined with the help of an apparatus called bomb calorimeter. It is made up of steel so that it can bear high pressure developed during the chemical reaction taking place in the calorimeter. The inner side of the steel vessel is coated with some non- oxidizable metal like Pt or Δu. It is also fitted with a pressure-tight screw-cap.
The two electrodes are connected to each other through a platinum wire dipped in a platinum cup.
A small known mass of the substance under investigation is taken in the platinum cup. The bomb is filled with excess of oxygen under a pressure of 20-25 atm and sealed. Now it is kept in an insulated water-bath which contains a known amount of water. The water bath is also provided with a thermometer and mechanical stirrer.
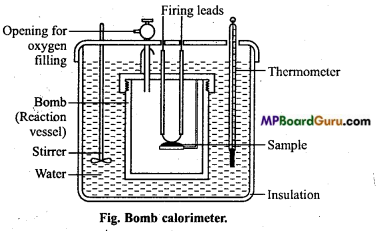
The initial temperature Of water is noted and the reaction (i.e., combustion of the sample) is started by passing an electric current through the Pt wire. The heat evolved during the chemical reaction raises the temperature of water which is recorded by the thermometer. When rise in temperature and the heat capacity of the calorimeter are known, the amount of heat evolved in the chemical reaction can be calculated. This will be equal to the change in internal energy (ΔE) of the reaction.
Calculation: Let W = Mass of calorimeter in gm,
w = Water equivalent of calorim-eter, bomb stirrer, etc.,
t °C = Rise in temperature,
x = Mass of compound ignited in gm and
m = Molecular mass of the compound.
Formula used : Heat produced by x gm compound = (W + w) t calories
∴ Heat produced by m gm compound = \(\frac{m}{x}\) (W + w)t calories
∴ Heat of combustion of the compound at constant volume,
ΔU or ΔE = – \(\frac{m}{x}\) (W + w)t calorie/mol.
Heat is evolved so negative sign is used.
Using equation ΔH = ΔE + ngRT, value of ΔH can be calculated at constant pressure.
![]()
Question 5.
Derive the expression for PV work.
Answer:
A cylinder containing one mole ideal gas is fitted with a weightless and friction-less piston of cross sectional area A. Total volume of gas is V and pressure is P and force F acting on the piston then :
P ressure = \(\frac{\text { Force }}{\text { Area }}\)
⇒ P =\(\frac{\mathrm{F}}{\mathrm{A}}\)
⇒ A × P = F ……………….. (i)
If force acting on the piston is 1ess than gas pressure then the piston moves up to a certain distance. Let this displacement be dl. As a result increase in volume is dV then work done by the system.
Work = Force × Displacement
W = F × dl
Substituting the value of F from eqn.(i),
W = P ×A × dl
⇒ W = Pdv,
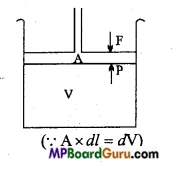
If increase in volume is from V1 to V2, then for determining , the total work it is integrated:
W = P\(\int_{V_{1}}^{v_{2}}\) dV
⇒ W= P(V2-V1)
W = PΔV
Use of Sign: If work is done by the system on the surroundings then work done is negative because for doing work internal energy of the system is used as a result of which internal energy decreases.
If work is done by the surroundings on the system, then work done is positive because there is an increase in the internal energy.
Question 6.
What is Free Energy? Discuss mathematical expression for it and derive Gibbs-Helmholtz equation.
Answer:
Free energy of a system is defined as the maximum amount of energy available to a system during a process which can be converted into useful work.
It is a state function introduced by Gibbs for considering energy and entropy change together. It is denoted by ‘G’. Mathematically, it is expressed as
G = H – TS
Where H = Enthalpy, T = Temperature in kelvin, S = Entropy.
Gibbs – Helmholtz equation: Consider a process taking place at constant temperature and constant pressure, enthalpy (H) of such a process is related with internal energy (U) as shown below:
H = U + PV ……………………. (i)
Where, P is pressure and V is volume of the system.
By definition, free energy, G is expressed as,
G = H – TS …………………………… (ii)
So G = (U + PV) – TS …………………… (iii)
∵ Absolute value of U and S cannot be determined, therefore, change in internal energy and entropy can be obtained as, ‘
ΔG = ΔU + Δ(PV) – Δ(TS)
or ΔG = ΔU + PΔV + YΔP – TΔS – SΔT ……………………………….. (iv)
∵ Process is carried out at constant temperature and constant pressure,
ΔT = 0, ∴ S ΔT = 0
and ΔP = 0, ∴ VAP=0 .
So, equation (iv) reduces to
ΔG = ΔU + PΔV – TΔS …………………. (v)
But ΔU + PΔV = ΔH
Then ΔG = ΔH-TΔS.
Alternatively : Gibbs equation G = H – TS can be written at initial and final states at constant pressure as follows :
G1 = H1-TS1 and G2 = H2 -TS2
ΔG = ΔG1 — ΔG2
or ΔG = (H2 -TS2) – (H1 -TS1)
or ΔG = (H2 -H1) – (TS2 – TS1) = ΔH-TΔS
Thus ΔG = ΔH-TΔS
This equation is known as Gibbs-Helmholtz equation.
Thermodynamics Class 11 Important Numerical Questions
Question 1.
In a process, 701J of heat is absorbed by a system and 394J of work is done by the system. What is the change in internal energy for the process? (NCERT)
Solution:
Given, q = +701J ( Heat is absorbed ∴ q is positive)
W = -394J ( Work is done by the system W is negative) By First Law of Thermodynamics,
Change in internal energy
ΔU = q + W
= +701J + (-394J) = + 307J
Thus, internal energy of the system increase by 307 J.
Question 2.
The reaction of cyanamide, NH2CN(s), with dioxygen was carried out in a bomb calorimeter and ΔU was found to be -742.7 kJ mol-1 at 298 K. Calculate enthalpy change for the reaction at 298 K (R = 8.314 × 10-3k JK-1mol-1). (NCERT)
NH2CN(s) + \(\frac{3}{2}\)O2(g) → N2(g)+ CO2(g) + H2O(l)
Solution:
NH2CN(s) + \(\frac{3}{2}\)O2(g) → N2(g)+ CO2(g) + H2O(l)
Difference between No. of moles of gaseous reactants and products,
Δng = np – nr = 2 – \(\frac{3}{2}\) = \(\frac{1}{2}\) = 0.5mol
∵ ΔH = ΔU + Δng RT
∴ ΔH = – 742 .7KJ mol-1 + (0.5 mol × 8.314 × 10-3 KJ K-1 mol-1 × 298 K)
ΔH = (-742.7 KJ + 1238.786 × 10-3 KJ ) mol-1
= -741.46 KJ mol-1
Question 3.
Calculate the enthalpy of formation of CH4 by the following data : (NCERT)
C(s) +O2(g) → CO2(g); ΔH = -97kcal …………………… (i)
2H2(g) + O2(g) → 2H2O(g); ΔH = -136k cal ……………….. (ii)
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g); ΔH =-212 k cal …………………………… (iii)
Solution:
To find out
2H2(g) + O2(g) → 2H2O(g) ΔH = ?
On adding eqns. (i) and (ii),
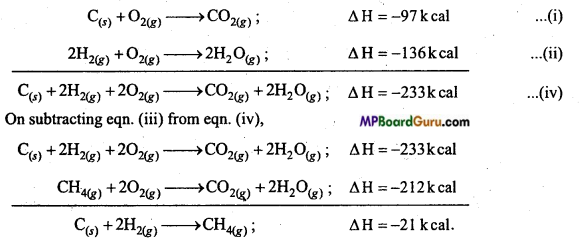
Question 4.
Calculate the number of kJ of heat necessary to raise the temperature of 60.0 g of aluminium from 3S°C to 55°C. Molar heat capacity of Al is 24 J mol-1 K-1. (NCERT)
Solution:
Given, Mass of Al = 60.0g
Molar mass of Al = 27g mol-1
Molar heat capacity Cm = 24J mol-1K-1
ΔT = 55°C – 35°C = 20°C or 20K
∴ Heat, . q = n.Cm. ΔT
q = \(\frac{60}{27}\) × 24 J mol-1 × 20K,
= 1066.66J = 1.067KJ (n = \(\frac{60}{27}\)mol-1).
![]()
Question 5.
Calculate the enthalpy change on freezing of 1.0 mole of water at 10.0° C to ice at -10.0°C. (NCERT)
ΔfusH = 6.03 KJ mol-1 at 0° C
Cp[H2O(l)] = 75.3 J mol-1 K-1
Cp[H2O(s)] = 36.8 J mol-1 K-1
Solution:
At 10°C, enthalpy change required to convert 1 mole liquid water to 1 mole liquid water at 0°C
ΔH, = Cp[H2O(l) ] × ΔT
= -75.3 J mol-1 K-1 × 10K = -753Jmol-1
Enthalpy of fusion ΔH2 = ΔHFreezing
= – ΔHFusion
= -6.03 KJ mol-1
Enthalpy change required to convert 1 mole ice at 0°C to 1 mole ice at 10°C
ΔH3 = Cp[H2O(s)] × ΔT = -36.8 J mol-1K-1-1 × 10K = -368 J mol-1
ΔHtotal = – (0.753 + 6.03 + 0.368) kJ mol-1
= – 7.151 kJ mol-1.
Question 6.
In a cooking gas cylinder 11.2 kg butane gas is Ailed. If a family requires 15,000 kJ energy everyday to cook food then for how many days will the gas be used.
Solution:
Equation of combustion of butane
C4H10(g)+\(\frac{13}{2}\) O2(g)→ 4CO2(g)+5H2O(g); ΔH = -2658kJ
Molecular mass of butane =12 × 4+ 10 × 1=58 gm/mol
By complete combustion of 58 gm butane 2658 kJ heat is released
Thus by 11.2 kg butane release heat= \(\frac{2658}{58}\) × 11.2 × 1000 KJ
Family requires 15,000 kJ of energy everyday
Thus, cylinder of 11.2 kg butane will last for = \(\frac{2658 \times 11 \cdot 2 \times 1000}{58 \times 15,000}\) = 34.2 days
Question 7.
Calculate the heat of formation of benzene with the following data :
C+O2 → CO2 , ΔH = 97 kcal ………………… (i)
H2 + \(\frac{1}{2}\) ΔH = 68 kcal ……………… (ii)
C6H6+\(\frac{15}{2}\)O2 → 6CO2 + 3H2O ΔH = 783.4 kcal …………………. (iii)
Solution:
Equation for heat of formation of benzene :
6C + 3H2 → C6H6; ΔH = ?
For calculating heat of formation of benzene multiply eqn. (ii) by 3 and add them and subtract eqn. (iii) from it. (i) by 6 and eqn.
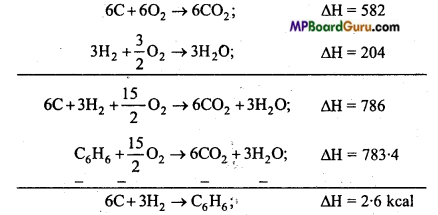
Question 8.
Enthalpy of combustion of carbon to CO2 is -393.5 kJ mol-1. Calculate the heat released upon formation of 35.2g of CO2 from carbon and dioxygen gas. (NCERT)
Solution:
Equation of combustion of carbon to CO2 is :
C(s) + O2(g) → CO2(g) ΔH = 393.5KJ mol-1
∴ Heat evolved in the combustion of 44g CO2 = 393.5KJ
∴ Heat evolved in the combustion of 35.2g CO2 = \(\frac{393 \cdot 5 \mathrm{~kJ} \times 35 \cdot 2 \mathrm{~g}}{44 \mathrm{~g}} \) = 314.8 KJ.
Question 9.
Given N2(g) +3H2(g) → 2NH3(g); ΔfH°= – 92.4kJ mol-1
What is the standard enthalpy of formation of NH3 gas? (NCERT)
Solution:
Given N2(g) +3H2(g) → 2NH3(g); ΔfH° = -92.4KJ mol-1
The following is the chemical equation for enthalpy of formation of NH3(g):
\(\frac{1}{2}\) N(2g) + \(\frac{3}{2}\) H(2g) → NH(3g)
Thus, ΔfH° = \(\frac{-92 \cdot 4}{2}\) = -46.2KJ mol-1.
![]()
Question 10.
A gas is compressed at an average pressure of 0.50 atm, by which its volume decreases from 300 cm3 to 100 cm3. Calculate the work done.
Solution:
Change in volume Δ V = 300 – 100 = 200 cm3 = 0.2 L, pressure = 0.5 atm , W = – PΔ V = – 0.5 × (- 0.2) = – 0.1 L atm.
= 0.1 × 101.3 J = 10.13 J.
Question 11.
If heat of combustion of diamond is-593.5 kJ and enthalpy of combustion of graphite is – 595.5 kJ, then determine the enthalpy of transition of diamond to graphite.
Solution:
Combustion of diamond is represented by the following reaction :
C(diamond) + O2(g) → CO2(g) ; ΔH = -593.5kJ ………………. (i)
and combustion of graphite is represented by the reaction :
C(graphite) + O2(g) → CO2(g); ΔH = -595.5 kJ……………… (ii)
Thus, Enthalpy of transition of diamond to graphite will be
C(diamond) → C(graphite) ΔH = ?
Above equation can be obtained by subtracting eqn. (ii) from eqn. (i),
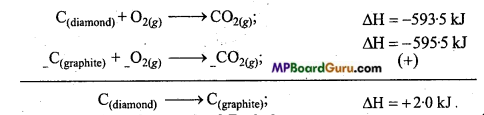
Question 12.
Calculate the standard Enthalpy of formation of CH3OH(l) from the following data:
Solution:
Required equation of manufacture of methanol:
C(s) +\(\frac{1}{2}\)O2(g) → CH3OH(l) ΔfH° =?
Given, Enthalpy of combustion of methanol
(i) CH3OH(l) +\(\frac{3}{2}\)O2(g) → CO2(g)+ 2H2O(l); ΔfH° = 726 KJmol-1
Enthalpy of formation of 1 mol CO2(g)
(ii) C(s) +O2(g) → CO2(g); ΔfH° = -393 KJ mol-1
Enthalpy of formation of 2 mole H2O(l)
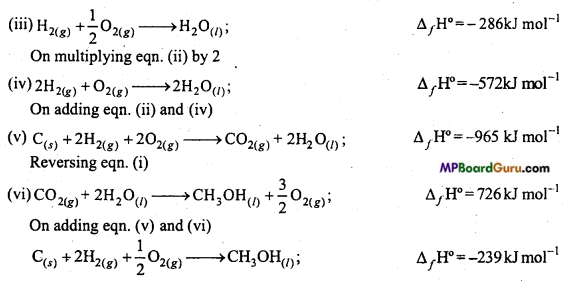
Question 13.
Calculate the enthalpy change for the process
CCl4(g) → C(g) + 4Cl(g) and calculate bond enthalpy of C – Cl in CCl4(g).
Given : Δ EvaporationH°(CCl4) = 30.5 kJ mol-1
ΔfH°(CCl4) = -135.5 kJ mol-1 Here ΔaH° is Enthalpy of atomization
ΔaH°(C) = 715.0 KJ mol-1
ΔaH°(Cl2) = 242 KJ mol-1
Solution:
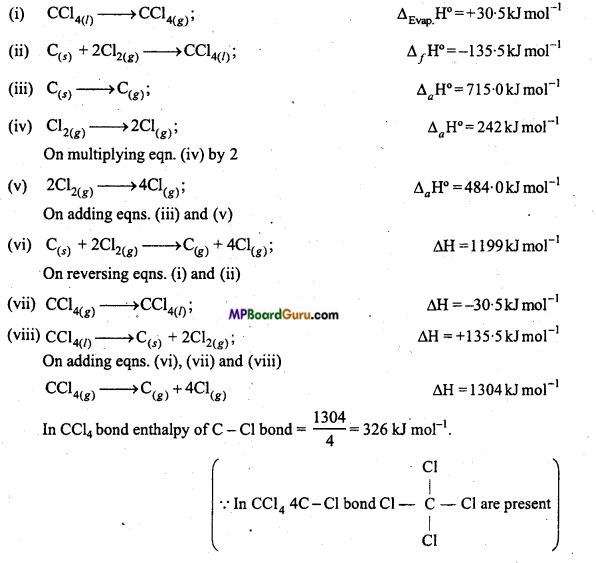
As given,
Question 14.
For the reaction at 298 K, 2A + B → C; ΔH = 400 kJ mol-1 and ΔS = 0.2 kJ K-1 mol-1
At what temperature will the reaction become spontaneous considering ΔH and ΔS to be constant over the temperature range. (NCERT)
Solution:
Given, ΔH = 400 kJ mol-1, ΔS = 0.2 kJ mol-1
Gibbs Free Energy ΔG = ΔH – TΔS
0 = 400 kJ mol-1 – T × 0.2 kJ K-1mol-1
∴ Temperature T = \(\frac{400 \mathrm{~kJ} \mathrm{~mol}^{-1}}{0 \cdot 2 \mathrm{~kJ} \mathrm{k}^{-1} \mathrm{~mol}^{-1}} \) = 2000K.
Thus at 2000K or above this temperature the reaction will be spontaneous.
![]()
Question 15.
Calculate the entropy change in surroundings when 1.00 mol of H2O(l) is formed under standard conditions. ΔfH° = – 286 kJ mol-1 kJ mol-1 (NCERT)
Solution:
Enthalpy change in formation (manufacture) of 1 mol H2O(l) ΔfH°= -286KJmol-1
H2(g) + \(\frac{1}{2}\)O2(g) → H2O(l);
Energy released in this reaction is absorbed by the surrounding,
Thus, qSurroundings = + 286 kJ mol-1
ΔS = \(\frac{q_{\text {surroundings }}}{T}\) = \(\frac{+286 \mathrm{~kJ} \mathrm{~mol}^{-1}}{298 \mathrm{~K}} \)
= 0.9597 KJ K-1 mol-1 = 959.7 JK-1 mol-1.
Question 16.
If 20.7 kJ of heat is producted by the combustion of 1 gm graphite, then what will be the molar enthalpy change. Clarify the concept of sings also. (NCERT)
Solution:
Molar enthalpy change AH for the combustion of graphite = Enthalpy of combustion of lgm graphite x Molar mass. *
ΔH = -20.7 kJg-1× 12gm mol-1
ΔH = – 2.48 × 102 kJ mol-1
ΔH represents negative value, therefore the reaction is exothermic.
Question 17.
Enthalpy of evaporation of CCl4 is 30.5 kJ mol-1. At constant pressure, calculate the heat required for the evaporation of 284gm CCl4. (Molar mass of CCl4= 154g mol1) (NCERT)
Solution:
∵ Mass of 1 mol CCl4 = 154gm
∴ ΔevaH for 154g CCl4 = 30.5 kJ
∴ ΔevaH for 284g CCl4 = \(\frac{30 \cdot 5 \times 284}{154}\) = 56.25 KJ.
Question 18.
State of 1 mole of a monoatomic gas undergoes expansion as shown in figure from state (1) To state (2) Calculate the work done at 298 K in expansion from state (1) to (2). (NCERT)
Solution:
It is clear from the figure that the process takes place in infinite steps. Thus at 298 K from 20 atm. to 1.0 atm it is isothermal reversible expansion of ideal gas.
W = -2.303RTlog\(\frac{\mathrm{P}_{1}}{\mathrm{P}_{2}}\)
W = -2.303 × 1 mole × 8.314 JK-1 mol-1×298 Klog2 [∵\(\frac{\mathrm{P}_{1}}{\mathrm{P}_{2}}\) = P]
W = -2.303 × 1 × 8.314 × 298 × 0.3010J
W = – 1717.46J.
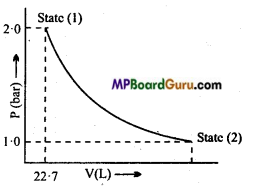
Question 19.
Calculate the ΔlatticeH° for NaBr by the help of the following data :
For Sodium metal ΔsubH° = 108.4 kJ mol-1
Ionisation energy of sodium = 496 kJ mol-1
Electron gain enthalpy of bromine =-325 kJ mol-1
Bond dissociation enthalpy of bromine = 192 kJ mol-1
ΔfH° for NaBr(s) = -360.1 kJ mol-1
Solution:
Born-Haber cycle for the manufacture of NaBr
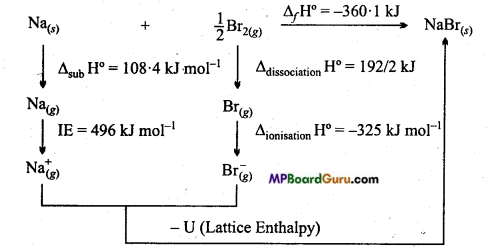
By Hess’s law
ΔfH° = ΔsubH° + IE +ΔdissocationH° + Δionsation H°+ U – 360.1
= 108.4 + 496 + 96 + (-325) – U
U = + 735.5 KJ mol-1
Thermodynamics Class 11 Important Questions Objective Type
1. Choose the correct answer:
Question 1.
A system is said to be a closed system, if it exchanges with the environment:
(a) Mass and energy both
(b) Neither mass nor energy
(c) Not mass only energy
(d) Not energy only mass.
Answer:
(c) Not mass only energy
Question 2.
The value of which enthalpy is always negative :
(a) Enthalpy of formation
(b) Enthalpy of solution
(c) Enthalpy of combustion
(d) Enthalpy of fusion.
Answer:
(c) Enthalpy of combustion
Question 3.
Reaction of H+ and OH– is named is :
(a) Hydrogenation
(b) Hydroxidation
(c) Hydrolysis
(d) Neutralisation.
Answer:
(d) Neutralisation.
Question 4.
A bomb calorimeter can measure:
(a) ΔH
(b) ΔE
(c) qp
(d) qv
Answer:
(d) qv.
![]()
Question 5.
At constant temperature and pressure, which statement is true for following reaction, CO(g) + \(\frac{1}{2}\) O2(g) → CO2(g) :
(a) ΔH = ΔU
(b) ΔH < ΔU (c) ΔH > ΔU
(d) ΔH × ΔV = 0.
Answer:
(c) ΔH > ΔU
Question 6.
According to the first law of thermodynamics :
(a) ΔE = q + W
(b) ΔE = q-W
(c) ΔE = q + PV
(d) W = ΔE – q.
Answer:
(a) ΔE = q + W
Question 7.
If in gaseous reaction number of moles of reactants and products is same then :
(a) ΔH = ΔE
(b) ΔH > ΔE
(c) ΔH < ΔE
(d) Δ H = Δ E = 0.
Answer:
(a) ΔH = ΔE
Question 8.
To which of the following is Hess’s law-related :
(a) Speed of reaction
(b) Equilibrium constant
(c) Change of Enthalpy of reaction
(d) By the effect of pressure on the volume of a gas.
Answer:
(c) Change of Enthalpy of reaction
Question 9.
Evaporation of water is :
(a) Endothermic change
(b) Exothermic change
(c) No change in enthalpy
(d) Process which occur with chemical reaction.
Answer:
(a) Endothermic change
Question 10.
In which process, there is no change in heat:
(a) Irreversible
(b) Reversible
(c) Adiabatic
(d) None of these.
Answer:
(c) Adiabatic
![]()
Question 11.
If Heat of neutralization of an acid and a base is 13.7 kcal then :
(a) Both acid and base are weak
(b) Both acid and base are strong
(c) Acid is strong and base is weak
(d) Acid is weak and base is strong.
Answer:
(c) Acid is strong and base is weak
Question 12.
In adiabatic expansion of an ideal gas always :
(a) Temperature increases
(b) ΔH = 0
(c) q = 0
(d) W = 0.
Answer:
(c) q = 0
Question 13.
Heat produced in the reaction CO2 (g)+H2(g) → CO2(g)+H2(g) is called:
(a) Heat of formation
(b) Heat of combustion
(c) Heat of neutralization
(d) Heat of reaction.
Answer:
(d) Heat of reaction.
Question 14.
In exothermic reaction :
(a) HR > Hp
(b) HR< HP
(c) HR = HP
(d)HR×HP = 0.
Answer:
(a) HR > Hp
![]()
Question 15.
Heat of neutralization is minimum for which reaction :
(a) HCl + NaOH
(b) CH3COOH + NH4OH
(c)NH4OH + HCl
(d) NaOH + CH3COOH.
Answer:
(b) CH3COOH + NH4OH
Question 16.
According to the reaction, C6H6(l)+\(\frac{15}{2}\)O2(g) → 3H2O(I)+6CO2(g) ΔH= 3264.6 KJ mol-1 The energy produced by burning 3.9 gm benzene in air will be :
(a) 163.23 kJ/mol
(b) 326.4 kJ/mol
(c) 32.64 kJ/mol
(d) 3.264 kJ/mol.
Answer:
(a) 163.23 kJ/mol
Question 17.
Which fuel has the highest calorific value (kJ/kg) :
(a) Charcoal
(b) Kerosene
(c) Wood
(d) Cow dung.
Answer:
(b) Kerosene
Question 18.
Heat of formation of N2O and NO are 82 and 90 kJ respectively. The enthalpy for the reaction
2N2O(g)+ O2(g) → 4NO(g)J will be:
(a) -16 kJ
(b) 196 kJ
(c) 98 kJ
(d) 88 kJ.
Answer:
(c) 98 kJ
Question 19.
Entropy of vaporization of a liquid is 100 JK-1 mol-1 and its boiling point is 300 K. ΔHVap for it will be :
(a) 3.0 kJ mol-1
(b) 30.0 kJ mol-1
(c) 300kJmol-1
(d) 3000 kJ mol-1.
Answer:
(b) 30.0 kJ mol-1
Question 20.
Enthalpy of atomization of CH4 is 1664 kJ mol-1, then bond energy of C-H will be: ,
(a) 416 kJ mol-1
(b) 832 kJ mol-1
(c) 1248 kJ mol-1
(d) -1664 kJ mol-1.
Answer:
(a) 416 kJ mol-1
Question 21.
Heat of formation of H2(g)+ I2(g) → 2HI(g)ΔH is 218 kJ mol-1. Bond energy of H-H will be :
(a) 218 kJ mol-1
(b) 436 kJ mol-1
(c) 109 kJ mol-1
(d) 6.54 kJ mol-1.
Answer:
(b) 436 kJ mol-1
Question 22.
In reaction H2(g) + kcal then heat of formation of (R)1H is:
(a) -124 kcal
(b) 124 kcal
(c) -620 kcal
(d) 620 kcal.
Answer:
(c) -620 kcal
Question 23.
A system is called closed system if it exchanges with the surrounding:
(a) Both matter and energy
(b) Neither matter nor energy
(c) Only energy not matter
(d) Only matter not energy.
Answer:
(c) Only energy not matter
Question 24.
Application of Hess’s law is:
(a) First law of Thermodynamics
(b) Second law of Thermodynamics
(c) Entropy change
(d) Free energy change.
Answer:
(a) First law of Thermodynamics
Question 25.
Relation between ΔG, AS and ΔH is:
(a) ΔH=tSG-TΔS
(b) ΔG=ΔH-TΔS
(c) ΔH = ΔG + ΔS
(d) ΔH= TΔG + ΔS.
Answer:
(b) ΔG=ΔH-TΔS
2. Fill in the blanks:
1. Value of heat of combustion ΔH is always …………………. .
Answer:
Negative
2. Value of heat of neutralization is always …………………. kJ.
Answer:
-57.1
3. Coffee kept in a tightly closed thermos flask is an example of …………………. .
Answer:
Isolated system
4. Total heat content of a system at constants pressure is called …………………. .
Answer:
Enthalpy
5. When a solid melts then …………………. increases.
Answer:
Enthalpy
6. Evaporation of water is a …………………. change.
Answer:
Endothermic
7. Value of Enthalpy of formation of the reaction H2+Cl2 → 2HCl+44 kcal is …………………. .
Answer:
22 kcal
8. System in which no heat is exchanged is called …………………. .
Answer:
Adiabatic
9. Mathematical expression of Thermodynamics is …………………. .
Answer:
ΔE = q + W
10. If total heat of the products is more than the total heat of the reactants then the reaction is …………………. .
Answer:
Endothermic
11. Heat change and constant pressure is called …………………. .
Answer:
Enthalpy
12. If ΔH is negative, then the reaction is …………………. .
Answer:
Exothermic
13. Amount of heat evolved by the combustion of one mole of substance is called …………………. .
Answer:
Heat of combustion.
![]()
3. Match the following:
| ‘A’ | ‘B’ |
| 1. Process of evaporation of water | (a) Zero |
| 2. Free energy change in equilibrium | (b) Isobanc |
| 3. Heat change at constant pressure | (c) Endothermic |
| 4. Heat of formation at 1 atmospheric. pressure and 25°C temperature is equal to | (d) Zero
|
| 5. Process in which pressure remain constant | (e) Enthalpy. |
Answer:
1. (c) Endothermic
2. (d) Zero
3. (e) Enthalpy
4. (a) Zero
5. (b) Isobanc.
4. Answer in one word/sentence:
1. Give two examples of result of state.
Answer:
Enthalpy, Entropy
2. Among NaCl, H2O(s) and NH3(q) whose entropy value will be maximum ?
Answer:
NH3
3. What is the heat of combustion of a substance ?
Answer:
Exothermic
4. ΔG = ΔH – TΔS who formulated this equation.
Answer:
Gibbs Helmholtz
5. Write the equation of first law of thermodynamics.
Answer:
AE = q + w
6. When ice melt and change into liquid, then what will be the value of entropy.
Answer:
Increases
7. Evaporation of water is what type of reaction ?
Answer:
Endothermic.