Students get through the MP Board Class 11th Chemistry Important Questions Chapter 7 Equilibrium which are most likely to be asked in the exam.
MP Board Class 11th Chapter 7 Equilibrium
Equilibrium Class 11 Important Questions Very Short Answer Type
Question 1.
While writing the expression for equilibrium constant explain why pure liquids and solids are neglected?
Answer:
Molar concentration of pure solid or liquid (if in excess) remains constant (i.e., independent of the amount present). That is why, while writing the expression for equilibrium constant pure liquids and solids can be neglected.
Question 2.
Of the following which is Lewis acid : H2O,BF3, H+,NH4+.
Answer:
Lewis acids gain electron. Electron deficient and positive charged species behave like Lewis acid.
BF3, H+,NH4+ behave like Lewis acids.
Question 3.
Write the formulae of conjugate bases for the following Bronsted acids :
HF, H2SO4 and HCO3–.
Answer:
Acid ⇌ Conjugate base + H+
HF ⇌ F– +H+ .
H2SO4 ⇌ HSO4 + H+
HCO3– ⇌ CO32- + H+.
Question 4.
What is active mass?
Answer:
Molecular concentration per litre of any solution or gas is called its active mass. It is represented by mol/litre. It is represented in square bracket [ ].
Active mass = \(\frac{\text { Mass of substance } / \text { Molecular mass of substance }}{\text { Total volume (in litre) }}\)
Question 5.
What do you understand by physical equilibrium? Explain with example.
Answer:
Physical equilibrium: When in a system, a substance exist in more than one physical state (solid, liquid, gas) and are changing into one another, then that stage in which the amount which changes from one form to another, the same amount gets converted to the first form is known as physical equilibrium.
Like, Water⇌ Water vapour (liquid-vapour equilibrium)
Ice ⇌ Water (solid-liquid equilibrium).
Question 6.
Explain Ostwald dilution law.
Answer:
Ostwald dilution law: Ostwald gave law for weak elecrolytes. By applying law of mass action Ostwald gave a law for expressing the dissociation of weak electrolyte. It states that:
“The degree of dissociation of weak electrolyte is directly proportional to the square root of its dilution.”
α = \(\sqrt{\mathrm{KV}}\) = \(\sqrt{\frac{\mathrm{K}}{\mathrm{C}}}\)
Where, α = Degree of dissociation, K = Dissociation constant, V = Volume in litre in which one gram equivalent is dissolved, C = No. of gram equivalent in one litre.
![]()
Question 7.
What is the effect of pressure on chemical equilibrium?
Answer:
On increasing the pressure on chemical equilibrium proceeds in that direction where there is decrease in volume i.e., number of molecules decreases.
Example: SO3 is formed by the combination of SO2 and O2 and 45.2 kcal heat is- released.
2SO2(g) + O2(g) ⇌2SO3(g); ΔH = – 45.2 kcal
In this reaction, two-volume SO2 reacts with one volume O2 to form two-volume SO3. Thus, on increasing the pressure, equilibrium shifts to forward direction.
Question 8.
What are buffer solution?
Answer:
Buffer solution: Thus, solution in which,
- pH value is definite.
- pH is not changed on dilution or on keeping for some time.
- On adding acid or base in less quantity, pH change is negligible.
Such solutions are called buffer solutions.
or .
Buffer solutions are solutions which retain their pH constant or unaltered.
Question 9.
Write acidic and basic buffer with one example each.
Answer:
1. Acidic buffers: Acidic buffers are formed by mixing an equimolar quantity of weak acid and its salt with a strong base.
Example: Acetic acid and sodium acetate (CH3COOH + CH3CQONa).
2. Basic buffers: Basic buffers contain equimolar quantities of weak base and its salt with strong acid.
Example: Ammonium hydroxide and ammonium chloride (NH4OH + NH4Cl).
Question 10.
State Le-Chatelier’s principle.
Answer:
Le-Chatelier’s principle: “If an equilibrium in a chemical system is disturbed by changing temperature, pressure or concentration of the system, the equilibrium shift in such a way so that the change gets minimised”.
This law is applicable for physical and chemical equilibrium.
Question 11.
What are the effects of catalyst at equilibrium?
Answer:
Effect of catalyst: Catalyst normally does not affect equilibrium, but helpful in establishing equilibrium soon catalyst affect both forward and backward reaction at the same rate. .
Actually by adding catalyst activation energy decreases and both forward and backward reactions are affected.
Question 12.
On the basis of eqn.pH = -log[H+], pH of 10-8 mol dm-3HCl solution should be 8. But its observed value comes to be less than 7. Explain the cause.
Answer:
10-8mol dm-3 concentration represents that the solution should be very dilute. Thus, we cannot neglect the H3O– ions produced by water. Thus, value obtained [H3O+] = (10-8 +10-7)M with this value pH of solution obtained is less than 7 (Because the solution is acidic).
Question 13.
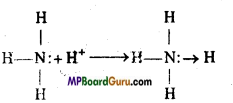
Ammonia is a Lewis base. Why?
Answer:
According to Lewis acid-base concept a base is a substance which can donate lone pair of electron. By the electronic structure of ammonia, it is clear that nitrogen con¬tains a lone electron pair which can be donated in a chemical reaction. Thus, ammonia behaves like a strong base.
Question 14.
In the precipitation of hydroxides of third group in place of NH4Cl and NH4OH, NaOH can be added in presence of NaCl or not?
Answer:
For the precipitation of hydroxides of third group, NH4OH is added in presence of NH4Cl due to common ion effect to supress the ionisation of NH4OH. NaCl + NaOH cannot be added in place of it because NaOH is a strong base and by common ion effect only ionisation of weak electrolytes is supressed. On adding NaOH hydroxides of further groups are also precipitated.
Question 15.
Write the use of buffer solution.
Answer:
Uses:
- In laboratories: In study of velocity of chemical reactions, buffers are used.
- Qualitative analysis: In removal of phosphate ion buffer of CH3COONa and CH3COOH is used.
- Industries: In production of alcohol by fermentation, buffers are used (pH between 5 to 6-8). In manufacturing of sugar, paper and in electroplating industries, buffers are used.
Question 16.
What is the effect of pressure and temperature in the solubility of gases in liquids?
Answer:
1. Effect of Pressure: On increasing the pressure, solubility of gases in liquid increases because molecules of gas occupy the intermolecular space of solvent.
2. Effect of Temperature: On increasing the temperature solubility of gas in liquids decreases because kinetic energy of molecules increases.
![]()
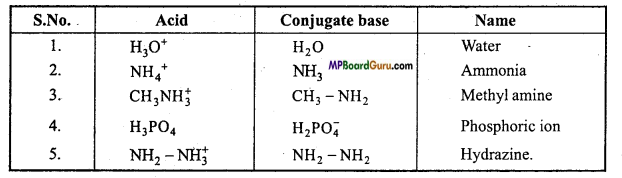
Question 17.
Write the names and formulae of conjugate base of the following acids :
(1) H3O+,
(2) NH4+,
(3) CH3NH+,
(4) H3PO4,
(5) NH2 – NH3.
Answer:
Q. 18. Write conjugate acid of the Bronsted bases NH2– NH3 and HCOO–.
Answer:
Base + H+ ≅ Conjugate acid
NH2– +H+ ≅ NH3
NH3 + H+ ≅ NH4+
HCOO– + H+ ≅ HCOOH.
Question 19.
Conjugate acid of a weak base is always strong. What will be the decreasing order of basicity of the following conjugate bases :
OH–,RO–,CH3COO-,Cl-.
Ans. Conjugate acids of the given bases are H2O, ROH, CH3COOH and HCl. Order of its acidity is as follows :
HCl > CH3COOH > H2O > ROH
Thus, order of basicity of their conjugate bases will be as follows :
RO– > OH– > CH3 COO– > Cl–.
Question 20.
What is the unit of Equilibrium constant?
Answer:
Unit of Equilibrium constant depends on the type of reaction. If there is no change in the number of molecules in a reaction then equilibrium constant has no unit, but if the number of molecules change during
a reaction then equilibrium constant has some unit. Example : N2(g) + 3H2(g) ≅ 2NH3(g)
Kc = \(\frac{\left[\mathrm{NH}_{3}\right]^{2}}{\left[\mathrm{~N}_{2}\right]\left[\mathrm{H}_{2}\right]^{3}}\)
Unit of Kc = \(\frac{[\mathrm{mol} / \mathrm{L}]^{2}}{[\mathrm{~mol} / \mathrm{L}][\mathrm{mol} / \mathrm{L}]^{3}}\)
= \(\frac{1}{[\mathrm{~mol} / \mathrm{L}]^{2}}\) [mol-2L-2.
Question 21.
Utility of pH value is more for the identification of acidic and basic solution. Why?
Answer:
By knowing the pH value of a solution we can determine whether the given solution is acidic, alkaline or neutral.
- If pH value is less than 7, then the solution will be acidic.
- If pH value is more than 7, then the solution will be basic.
- If pH value is 7, then the solution is neutral.
Question 22.
Name the factors which affect chemical equilibrium.
Answer:
Factors which affect chemical equilibrium are :
- Temperature
- Pressure
- Concentration change
- Catalyst.
Question 23.
Species H2O, HCO3, HSO4 and NH3 behave both as Bronsted acid and base. Tell the conjugate acid and base of both.
Answer:
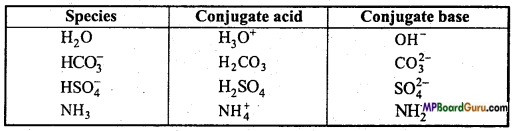
Question 24.
What is the pH value of pure water and drinking water?
Answer:
Pure water is neutral because number of H+ ion and OH– ion present in water is same and ionic product of water is 1 × 10-14 and hydrogen ion concentration is 1 × 10-7gram ion per litre and pH value is 7 and pH of drinking water is less than 7.
Question 25.
What do you mean by ionization of water?
Answer:
Ionization of water: Water is self ionized to some extent in the following way:
H2O + H2O ≅ H3+ + OH–
Equilibrium constant K = \(\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{H}_{2} \mathrm{O}\right]^{2}}\)
Since water is in excess, its concentration is supposed to be constant and product of it with K is another constantKw.
K × [H2O]2 = [H3O+ ][0H–]
or Kw = [H3 O+][OH–] = 1 × 10-14, (at 298K)
Where, Kw is called ionic product of water.
![]()
Question 26.
Hydrogen ion concentration in water is 10–7 gram ion per litre still water is neutral why?
Answer:
In pure water, concentration of hydrogen and hydroxyl ion is same.
Thus, in pure water
[H3 O+] = [OH–] = 1 × 10 -7 gram ion per litre.
Therefore pure water is neutral.
Question 27.
Write the formula expressing relation between concentration and pres-sure equilibrium constant. Or, Write the relation between Kp and Kc
Answer:
Relation between concentration and pressure equilibrium constant
Kp = Kc× RTΔn
Where Kp = Pressure equilibrium constant.
Kc= Equilibrium constant.
R = Gas constant, T = Absolute temperature.
Δn = Difference between number of moles of products and reactants.
Equilibrium Class 11 Important Questions Short Answer Type
Question 1.
Aqueous solution of sodium carbonate is basic. Why?
Answer:
Na2CO3 ⇌ 2Na+ + CO3 -2
2H2O ⇌ 2H++2OH–
Na2CO3 + 2H2O ⇌ 2NaOH + H2CO3
Strong Weak .
base acid
Na+ ion obtained by Na2CO3 combines with 0W to form strong electrolyte NaOH
due to which it remains in the form of ions whereas CO3 -2 ions combine with H ions to form H2CO3 which being a weak electrolyte remains partially ionized. To maintain the equilibrium, H2 ionizes and concentrat on of OH– ion increases, therefore aqueous solution of sodium carbonate is basic.
Question 2.
What is the effect on equilibrium while dissolving gases in liquids? Explain with example.
Answer:
Dissolution of gases in liquids: When the soda water bottle is opened, dissolved CO2 gas in it comes out rapidly. This is an example of equilibrium of any gas in equilibrium between gas and its liquid. At fixed pressure of the gas, there exists an equilibrium between soluble and insoluble molecules of the gas.
C02(g) ⇌ CO2(In solution)
In this way, William Henry gave a law for equilibrium and which is called Henry’s law.
Henry’s law: The solubility of gas in a given solvent iš directly proportional to the
pressure to which the gas is subjected, provided the temperature remains the same.
Thus, m ∝ P
or m=KP
Where K is a constant of proportionality and known as Henry’s constant. At the time of closing soda water bottle, pressure inside the bottle is very high as compared to atmospheric pressure, due to which in this state more gas is dissolved in the solution. At the time of opening the bottle, pressure acting on the solution becomes suddenly very less (equal to atmospheric pressure) so to establish new equilibrium, more quantity of gas comes out of the bottle.
![]()
Question 3.
What is common ion effect? Explain.
Answer:
The supression of ionization of weak electrolyte by the addition of strong electrolyte containing a common ion is known as common ion effect. For example, when CH3 COONa is added to CH3COOH solution, dissociation of CH3COOH decreases.
Example: In the identification of second group HCl + H2S is used as group reagent.
Solubility product of sulphides of group IIs comparatively less than the solubility product of sulphides of group IV. On passing H2S in the presence of HCl, due to common ion effect ionisation of H2S decreases due to which concentration of sulphide ions decreases in the solution.
HCl ⇌ H+ + Cl–
H2S ⇌ 2H+ + S2-
Thus, ionic product of sulphides of group IV does not exceed their solubility product but concentration of sulphide ions is sufficient for sulphides of group two because solubility product of sulphides of group II is less.
Question 4.
What are the characteristics of Equilibrium constant?
Answer:
1. At a definite temperature, for a reaction, value of equilibrium constant has a definite value. Its value changes with the change in temperature.
2. Value of equilibrium constant of a reaction does not depend on pressure and volume.
3. Value of equilibrium constant is independent of initial concentration of reactants and products but depend on their concentration in equilibrium.
4. If the reaction is reversed, the value of equilibrium constant is the inverse of the previous reaction.
5. If a chemical reaction is divided by 2, then the equilibrium constant for the new reaction will be the square root of equilibrium constant of the known reaction before
K’ = \(\sqrt{\mathrm{K}}\)
6. If a chemical reaction whose equilibrium constant is K, is multiplied by 2 then the equilibrium constant (K) obtained for the new reaction will be the square of K.
K’ = K2
7. If the reaction is written in two steps and equilibrium constants are K, and K2 of these reactions, then
K = K1 × K2.
Question 5.
Explain Homogeneous and Heterogeneous equilibrium with examples.
Answer:
Homogeneous equilibrium reactions: Such reactions in which reactants and products all are in the same physical states are called homogeneous equilibrium reactions.
Example:
- H2(g) + I2(g) ⇌ 2HI(g) (Δn = 0)
- N 2(g) + 3 H2(g) ⇌ 2NH3(g) (Δn=-2)
- PCl5(g) ⇌ PCl 3(g) + Cl2(g) (Δn= 1)
- CH3COOH(l) + C2H5OH(l) ⇌ CH3COOC2H5 (l)+H2O(l) (Δn = 0)
2. Heterogeneous equilibrium reactions: Such reactions in which reactants and products exist in one or more physical states are called heterogeneous equilibrium reactions. Example:
- 3Fe(s)+4H2O(g) ⇌ Fe3O4(s) + 4H2(g)
- CaCO3(s) ⇌ CaO(s) + CO2(g)
- 2Na2O2(s) + 2H2O(l) ⇌ 4 NaOH (aq) + O2(g)
Question 6.
Comparing the values of Kc and Qc how will you determine the following stages of a reaction :
(i) Resultant reaction proceeds in the forward direction.
(ii) Resultant reaction proceeds in the backward direction.
(iii) No change takes place in the reaction.
Answer:
(i) If Qc < Kc; reaction proceeds in the direction of products (Forward reaction).
(ii) If Qc > Kc.; reaction proceeds in the direction of reactants (Backward reaction).
(iii) If Qc = Kc; reaction mixture remain unchanged in equilibrium. Thus, no net change occur in the reaction.
Question 7.
Of the following in each equilibrium when volume is increased and pres-sure is decreased, then state whether the number of moles of products of the reaction increases or decreases or remains same :
(a) PCl5(g) ⇌ PCl3(g) +Cl2(g)
(b) CaO(s) +CO2(g) ⇌ CaCO3(g)
(c) 3Fe(s) + 4H2 O(g) ⇌ Fe3O4(s)+4H2(g).
Answer:
According to Le-Chatelier’s principle: On decreasing the pressure, equilib¬rium displaces in the direction where pressure increases, (i.e., in gaseous state, number of moles increases). Thus, number of moles of products of reaction :
(a) Increases
(b) Decreases
(c) Remains unchanged (If Δng = 0, then there will be no effect of change in pressure).
![]()
Question 8.
What is meant by concentration ratio?
Answer:
Ratio of concentrations of products and reactants is known as concentration quotient. For a reversible reaction concentration ratio Q is equal to its equilibrium constant Kc.
Kc = \(\frac{[\mathrm{C}]^{c}[\mathrm{D}]^{d}}{[\mathrm{~A}]^{a}[\mathrm{~B}]^{b}}\)
Q=\(\frac{\left[\mathrm{C}_{\mathrm{C}}\right]^{c}\left[\mathrm{C}_{\mathrm{D}}\right]^{d}}{\left[\mathrm{C}_{\mathrm{A}}\right]^{a}\left[\mathrm{C}_{\mathrm{B}} p\right.} \)
Question 9.
Explain ionisation .equilibrium with example.
Answer:
Whenever an ionic compound is dissolved in water or in a suitable solvent it ionizes into cation and anion. This process of ionization of an ionic compound into ions is called ionization at the compounds are called electrolytes. Ionic compounds which ionize completely are called strong electrolytes. Like NaCl, NaOH, H2SO4 etc.
alternatively compounds which do not ionize completely are called weak electrolytes like NH4OH, CH3COOH etc. When weak electrolytes are dissolved in H2O then in the solution an equilibrium is established between ions and unionised molecules which in called ionization equilibrium.
∴ NH4OH (aq) ⇌ NH+4(aq) + OH– (aq)
Question 10.
What do you understand by chemical equilibrium? Write its characteristics. Or, Write any four characteristics of chemical equilibrium.
Answer:
Chemical equilibrium: Chemical equilibrium of any reversible reaction is that state in which there is no change of concentration of the reactant and products take place.
Characteristics of chemical equilibrium :
- Velocity of forward and backward reaction is same.
- At the equilibrium mass of reactants and products remain the same.
- Equilibrium can be changed by changing temperature, pressure and concentration.
- The equilibrium is characterized by the kinetic energy. Reaction does not cease.
- At equilibrium, free energy change is zero, i.e., ΔG = 0.
Question 11.
Which of the following reactions will be affected on increasing the pressure? Also state, that on increasing the pressure, the reaction will proceed in forward or backward direction :
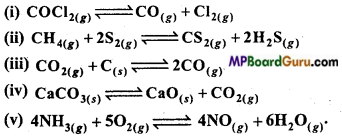
Answer:
(i) np > nr will proceed in backward direction.
(ii) np = nr due to increase in pressure equilibrium will not be affected.
(iii) np > nr will proceed in backward direction.
(iv) np > nr will proceed in backward direction.
(v) np > nr will proceed in backward direction.
Question 12.
What is meant by physical equilibrium? Write its main characteristics.
Answer:
Equilibrium which is established by physical changes is called physical equilib¬rium. In other words equilibrium which is established between two stages of a single compound and there is no change in the chemical composition .of the compound during the reaction but only there is a change in its physical state is called physical equilibrium.
Example: Ice(s) ⇌ Water(l)
Ice(s)⇌ Water vapour(g)
Characteristics of physical equilibrium:
- System should be closed or there should not be any mutual exchange with the surroundings.
- Equilibrium is dynamic but the stable conditions exist. Both process proceeds with same velocities.
- Concentrations of substances remain constant. Due to which quantities for measurement of system also remain constant.
- As the equilibrium is established concentrations of reactants are expressed by a relation which has a definite value at constant temperatures.
Question 13.
Define solubility product and explain it.
Answer:
Solubility product: The product of the concentration of ions in the saturated solution of a sparingly soluble salt as AgCl is a constant at a given temperature is called solubility product. If a sparingly soluble electrolyte at any temperature forms saturated solution then the equilibrium is
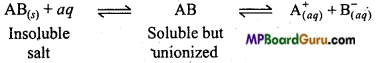
∴ According to law of mass action,
K = \(=\frac{\left[\mathrm{A}_{(a q)}^{+}\right]\left[\mathrm{B}_{(a q)}^{-}\right]}{\left[\mathrm{AB}_{(s)}\right]} \)
or K [AB(s)] = [A+aq] [B,sup>-aq]
or Ksp = [A+aq [B–aq]
When solution is saturated [AB(s)] will be constant and value of K[AB(s)] will also be a constant and is written as Ksp which is the solubility product.
Question 14.
Establish a relation between the solubility and solubility product of a sparihgly solution binary electrolyte.
Answer:
Relationship between solubility and solubility product: Suppose, AB is bivalent electrolyte whose solubility is S gm mol per litre. Solubility of sparingly soluble salt is very little, therefore, its saturated solution will also be very dilute. If AB is a strong
electrolyte, it will be ionized completely therefore, concentration of A+and B~ ions will be S mol/litre.
i.e., AB ⇌ A++B–
or [A+][B–] = Ksp .
or [S][S] = Ksp
or S2 =Ksp
∴ S = \(\sqrt{\mathrm{K}_{s p}}\)
Therefore, “Solubility of any sparingly soluble bivalent electrolyte is equal to the square root of the solubility product.”
![]()
Question 15.
Explain solubility product with the example of AgCl.
Answer:
AgCl(s) + aq ⇌ Agaq+ + Claq–
According to Law of Mass action
K = \(\frac{\left[\mathrm{Ag}_{a q}^{+}\right]\left[\mathrm{Cl}_{a q}^{-}\right]}{\left[\mathrm{AgCl}_{(s)}\right]}\)
[KAgCl(s)= Ksp]
⇒ Ksp = [Agaq+][Claq–]
Thus, in a saturated solution of AgCl, product of concentration of Ag+ and Cl– ions is the solubility product.
Question 16.
What is meant by Lewis acid and Lewis base? Explain with example.
Answer:
Lewis acid: Such molecules, ions or radicals which require a lone pair of electrons to complete the octet of their central atom are called Lewis acids. Thus, lone electron pair acceptor are called Lewis acids.
Example : BF3, AlCl3, Br+, NO2 etc.
Lewis base: Such molecules, ions or radicals whose central atom has a complete octet and can donate lone electron pair in a chemical reaction to for a co-ordinate bond is called Lewis base. Thus lone electron pair donor are called Lewis bases.
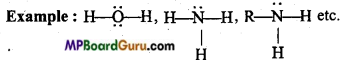
Question 17.
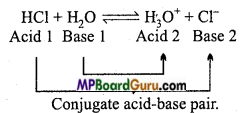
What do you understand by conjugate acid and conjugate base?
Answer:
Conjugate acid: When a molecule or ion gains a proton then the group formed as an acid, because it has the tendency to donate a proton is called conjugate acid of the base.
Example: Conjugate acid of NH3 is NH4+
Conjugate base: When a molecule, ion or acid donates a proton then the remaining group acts as a base because it possesses the tendency to gain a proton. This is known as conjugate base of the acid.
Example: Conjugate base of HC1 is Cl–.
Thus, conjugate acid and conjugate base differ by a proton. Conjugate base of^strong acid is always weak. „
Question 18.
At 310 K, ionic product of water is 2.7 × 10-14. Determine the pH of neutral water at this temperature.
Answer:
Kw = [H3O+].[OH–] = 2.7 × 10-14 (At 310 K)
For the reaction H2O + H2O ⇌ H3O++ OH–
[H3O+] = [OH–]
Thus, [H3O]= \(\sqrt{2 \cdot 7 \times 10^{-14}}\) = 1.643× 10-7M
pH = – log[H3O] = – log 1.643 × 10-7
pH = 7 + (-0.2156) = 6.7844.
Question 19.
What is pH scale? Establish its relation with hydrogen ion concentration.
Answer:
To express the acidity or basicity of a solution Sorensen in 1909 established a scale known as the pH scale.
The pH value of a solution is the numerical value of the negative power to which 10 should be raised in order to express the hydrogen ion concentration of the solution, i.e.,
[H+] = 10-pH
On taking log of eqn. (i),
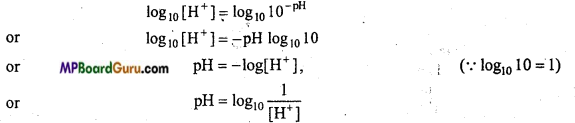
This is the required relationship between pH and H+ ion concentration.
Thus, the pH value of a solution is the logarithm of its hydrogen ion concentration to base 10 with negative sign.
pH scale expresses acidic and basic nature of solution in terms of numbers from 0 to 14. Acidic solutions have pH value less than 7 while basic solutions have pH value greater than 7. pH value of neutral solution is 7.
Question 20.
Before passing H2S, the filterate of first group is acidified by HCl. Why?
Answer:
Filterate of first group is used for the identification of sulphides of second group for which before passing H2S the filterate is acidified by HCl. Solubility product of sulphides of second group is less than the solubility product of sulphides of fourth group.
H2S is a weak electrolyte which partially ionizes, but HCl is a strong electrolyte, then due to common ion effect the ionization of H2S is further decreased as a result of which concentration of sulphide ion decreases. Due to this, the ionic product of fourth group metal sulphides do not exceed their solubility product, therefore they do not precipitate, but concentration of sulphide ions is sufficient for the precipitation of sulphides of second group because their solubility product is less.
![]()
Question 21.
Tell the maximum concentration of isomolar solutions of FeSO4 and Na2S when on mixing their similar volume iron sulphide is not precipitated (For iron sulphide
Ksp = 6.3 × 10 -18
Solution:
At precipitation point [Fe2+ ] [S2- ] = Ksp
[Fe2+] = [S2-] = \(\sqrt{\mathrm{K}_{s p}}\) = \(\sqrt{6 \cdot 3 \times 10^{-18}} \)
[Fe2+] = [S2-]] = 2.51 ×10-9 M
Since, similar volume of solutions are mixed, thus concentration of each solution re¬mains half, due to which in the basic solution
[FeSO4 ] = [Na2S] = 2 × 2.51 × 10-9M = 5.02 × 10–9 M
Thus, highest molarity of the solution = 5.02 x 10-9 M.
Question 22.
If pH of pyridinium hydrogen chloride solution is 0.02M, then determine the ionisation constant of pyridine.
Solution:
Pyridinium hydrogen chloride is the salt of a weak base (Pyridine) and strong acid HCI. .
C6H5N+HCl + H2O ⇌C6H5N+HOH–+HCl
Thus, pH = \(-\frac{1}{2}\) [logKw -logKb+logC]
(Due to hydration, solution is acidic)
3.44 = \(-\frac{1}{2}\)[-14-logKb + log 2.0 × 10-2]
or 6.88 = 14 + logKb+1.70
or log Kb = – 8.82 = \(\overline{9}\).18
or Kb =anti log \(\overline{9}\).18 = 1.5 × 10-9.
Question 23.
Reaction of NaCl with water is not counted in hydrolysis. Why?
Answer:
NaCl does not hydrolyse because when NaCl is dissolved in water it ionizes to form Na+ and Cl– ions. Na+ ion cannot combine with OH– ion because NaOH is a strong electrolyte. Similarly Cl– ion does not combine with H+ ion because HCI is a strong electrolyte.
This way in aqpeous solution, concentration of H+ and OH– ions is equal. Thus, aqueous solution of NaCl neither acts as an acid nor a base, therefore it is not counted in hydrolysis reaction.
NaCl + H2O ⇌ NaOH + HCl
Question 24.
Write the concentration ratio of the following reactions :
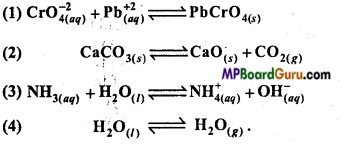
Answer:
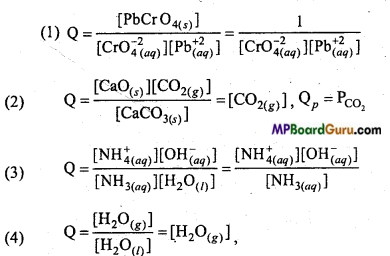
Qp = PH2O.
Question 25.
Ionisation constant of chloroacetic acid is 1.35 × 10-3. Calculate the pH value of 0.1M and its 0.1M sodium salt.
Solution:
CH2ClCOOH + H2O ⇌CH2ClCOO– + H3O+
Given, Ka=1.35 ×10-3
Sodium salt of chloroacetic acid is formed by a strong base NaOH and weak acid chloroacetic acid.
Thus, for a salt formed by a strong base and weak acid
pH=\(-\frac{1}{2}\) [log Kw + loga – log C]
or pH = 7 + \(\frac{\mathrm{pK}_{a}+\log \mathrm{C}}{2}\) = 7 +\(\frac{2 \cdot 87+\log 0 \cdot 1}{2}\)
= 7 + 0.935 = 7.94
pH = 7.94
Question 26.
Identify the neutral, acidic and basic nature of aqueous solutions of the following salts:
NaCI, KBr, NaCN, NH4NO3, NaNO2 and KI.
Ans.
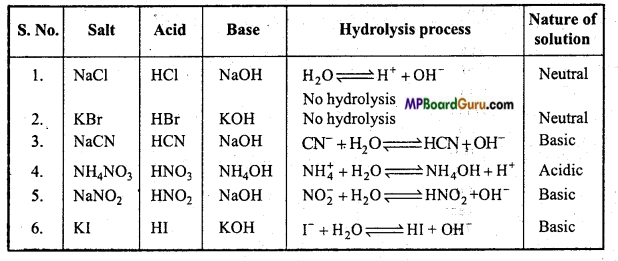
Question 27.
Write equilibrium constant expression for the following reactions:
1. BaCO3(s) ⇌ BaO(s)+CO2(g)
2. CH3COCH3(l) ⇌ CH3COCH3(g)
3. AgBr(s) + aq ⇌ Ag+aq + Br–aq
4. CH4(g) + 2O2(g) ⇌ CO2(g) + 2H2O(l).
Answer:
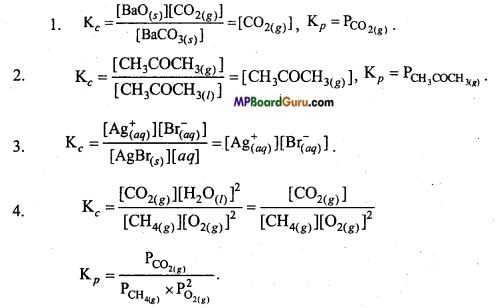
Question 28.
What is the importance of solubility product in the purification of common salt?
Answer:
The phenomenon used for purification of sodium chloride is known as salting out. For doing this a saturated aqueous solution of impure sodium chloride is prepared. Ahead equilibrium exists in it:
NaCl(s) ⇌ Na+ + Cr –(In solution)
Ksp = [Na+][Cr–]
When HCl gas is passed through this solution, the concentration of Cl– ions increases considerably. Due to this ionic product exceeds the solubility product of NaCl. Therefore, NaCl precipitates out from the solution in pure state while impurities remain in the solution.
Question 29.
Solubility of AgCI in water is more than in the solution of common salt. Why?
Answer:
When a partially soluble salt does not form corresponding salt with common ion, then in presence of common ion solubility of salt decreases. Since ionic product of the salt is more than its solubility product, solubility of AgCI is more in water than in salt solution. In presence of NaCl, concentration of ions increases in solution due which value of ionic product exceeds its solubility product by which the salts start precipitating and its solubility decreases.
Question 30.
What is the importance of solubility in the precipitation of soap?
Answer:
Soaps are sodium or potassium salts of higher fatty acids which are obtained by the hydrolysis of oils or fats by base. In hot process of manufacture of soap, soap is obtained in the form of a concentrated solution in which saturated solution of common salt is added for its precipitation. On adding concentrated solution of common salt, concentration of Na+ion increases by which value of ionic product exceeds the solubility product of soap at that temperature and solid soap is precipitated in its solution.
![]()
Question 31.
For the precipitation of hydroxides of third group using ammonium hy-droxide, ammonium chloride is added, why?
Answer:
Radicals of third group (Fe3+, Al3+, Cr3+) are precipitated as hydroxides. For this NH4Cl is added first followed by NH4OH. Addition of NH4Cl suppresses the dissociation of weak electrolyte NH4OH due to common ion effect. Thus decreases the number of OH– ions and hence only radicals of III group are precipitated as hydroxides.

If NH4OH is added without addition of NH4Cl then concentration of OH– ions in solution is very high due to which hydroxides of IV, V and VI groups get precipitated along with Fe, Cr and Al
Question 32.
Why is high temperature more favourable for the synthesis of nitric oxide than for ammonia ?
Answer:
N2+O2 ⇌2NO; ∆H = + 43kcal
N2 +3H2 ⇌ 2NH3 ; ∆H = -92.4kJ
In the above reactions, synthesis of nitric oxide is endothermic and synthesis of ammonia is exothermic. In the synthesis of nitric oxide, high temperature will favour the reaction i. e., there will be increase in the production of NO.
According to Le-Chatelier’s principle, whereas in case of synthesis of NH3, high temperature will favour backward reaction which will decrease the production of NH3. That is why high temperature is more favourable for the synthesis of NO.
Question 33.
At 298 K solubility of Sr(OH)2 solution is 19.23 gm/L. Determine the concentration of strontium and hydroxyl ion and pH of the solution.
Solution:
Solubility of Sr(OH)2 = 19.23gm/L (At 298 K)
Molarity (M) =\(\frac{19 \cdot 23(\mathrm{gm})}{121 \cdot 6 \mathrm{gm}^{5} \mathrm{~mol}^{-1} \times 1 \mathrm{~L}} \)
(Molecular mass of Sr(OH)2 = 87.6 +2(16 + 1) = 121.6gmol-1)
or M = 0.1581 mol L-1
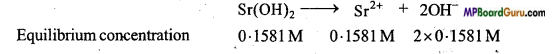
[Sr2+] = 0.1581M
[OH–] = 0.3162M
[H+].[OH] = 10-14 (Kw = 10-14 = [H+].[OH–])
or [H+] = \(\frac{10^{-14}}{0 \cdot 3162}\) = 3.16 × 10-14.
∴ pH = – log[H+] = – log[ 3.16 × 10-14]
or pH = 14 – 0.4997 = 13.5003 = 13.5
Question 34.
Explain Bronsted and Lowry Acid-Base Concept with example.
Answer:
Bronsted and Lowry concept: According to this theory, acid is a substance which donates a proton whereas base is a substance which accept a proton.
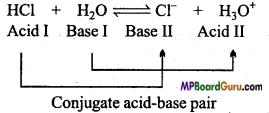
In this example HCl donates a proton therefore it is an acid whereas H2O is a proton acceptor, so H2O is a base. When an acid donates a proton the remaining group acts as a base and it is known as conjugate base of the acid. Similarly, when a base accepts a proton then the group formed acts as an acid and it is known as conjugate acid of the base. Conjugate acid-base differ by only one proton.
Question 35.
Calculate equilibrium constant for N2(g) +O2(g)⇌ 2NO(g).
Answer:
Rate of forward direction ∝ [N2][O2]=K1[N2][O2]
Rate of backward direction ∝ [NO]2=K2 [NO]2
At equilibrium Kc = \(\frac{\mathrm{K}_{1}}{\mathrm{~K}_{2}}\) = \(\frac{[\mathrm{NO}]^{2}}{\left.\mathrm{~N}_{2}\right]\left[\mathrm{O}_{2}\right]}\)
Question 36.
Only define the following :
(1) Salt hydrolysis
(2) Solubility product
(3) Common ion effect
(4) Buffer solution.
Answer:
1. Salt hydrolysis: Salt hydrolysis is the process, in which the cation or anion reacts with water to form base or acid as a result of which the nature of solution becomes acidic or basic.
2. Solubility product: The ionic product of concentration of ions in the saturated solution of an electrolyte is known as its solubility product.
3. Common ion effect: The supression of ionization of a weak electrolyte in the presence of a strong electrolyte containing a common ion is called common ion effect.
4. Buffer solution : Solutions which show a negligible change in their pH value on addition of a small amount of an acid or a base are called Buffer solution.
![]()
Question 37.
Give name and formulae of the following compounds along with one-one example:
(a) Salt formed by a strong acid and strong base.
(b) Salt formed by a weak acid &nd strong base.
(c) Salt formed by a strong acid and weak base.
(d) Salt formed by a weak acid and weak base.
Answer:
(a) Example of salt formed by a strong acid and a strong base: NaCl (Sodium chloride).
(b) Example of salt formed by a weak acid and a strong base: CH3COONa (Sodium acetate).
(c) Example of salt formed by a strong acid and weak base: NH4Cl (Ammonium chloride).
(d) Example of salt formed by a weak acid and a weak base: CH3COONH4 (Ammonium acetate).
Equilibrium Class 11 Important Questions Long Answer Type
Question 1.
Establish the relation between equilibrium constant Kp and Kc. Or, Prove that Kp =Kc. RTΔn.
Answer:
Guldberg and Waage, two Norwegian chemists recognized the relationship between active mass or molar concentration of reactants and rate of reaction. On the basis of observed relationship they formulated a law known as law of mass action. This law states that “the rate of reaction is directly proportional to the product of the concentration of the reactants with each concentration term raised to the power equal to the number of moles of the reactants involved in the balanced chemical equation.” Thus, for a general reaction in which reactant A reacts to form product, i.e.,
A → Product –
Law of mass action can be written as Rate ∝ [A] or
Rate = K[A]
Where, [A] represents molar concentration of reactant A and k is a constant called rate constant. Similarly, for a hypothetical reaction
aA + bB → Products
The law of mass action is – Rate ∝[A]a[B]b
or Rate = k[A]a[B]b
Where, [A] and [B] represent molar concentration of reactants A and B while a and b are their moles and k is the constant known as rate constant or velocity constant. Relation between Kp and Kc:
Consider a gaseous reaction is written as
n1A + n2B ⇌ m1 C+m2 D
On applying the law of mass action and if the concentrations are expressed in mol/litre.
i.e., ”
Kc = \(\frac{[\mathrm{C}]^{m_{1}} \times[\mathrm{D}]^{m_{2}}}{[\mathrm{~A}]^{n_{1}} \times[\mathrm{B}]^{n_{2}}} \)
or Kc = \(\frac{\mathrm{C}_{\mathrm{C}}^{m_{1}} \times \mathrm{C}_{\mathrm{D}}^{m_{2}}}{\mathrm{C}_{\mathrm{A}}^{n_{\mathrm{A}}} \times \mathrm{C}_{\mathrm{B}}^{n_{2}}} \) ……………….. (i)
or Where ,C = Molar concentatration.
If the partial pressure of reactants and products are represented, then equilibrium constant will be expressed by kp. i.e.,
Kp = \(\frac{\mathrm{P}_{\mathrm{C}}^{m_{1}} \times \mathrm{P}_{\mathrm{D}}^{m_{2}}}{\mathrm{P}_{\mathrm{A}}^{n_{1}} \times \mathrm{P}_{\mathrm{B}}^{n_{2}}} \) ………………….. (ii)
According to ideal gas equation, PV = nRT
P = \(\frac{n}{\mathrm{~V}}\) RT = CRT …………………. (iii)
Putting the value of P from eqn. (iii) in eqn. (ii),
Kp = \(\frac{\left(C_{C} R T\right)^{m_{1}} \times\left(C_{D} R T\right)^{m_{2}}}{\left(C_{A} R T\right)^{n_{1}} \times\left(C_{B} R T\right)^{n_{2}}}\)
= \(\frac{C_{\mathrm{C}}^{m_{1}} \times C_{\mathrm{D}}^{m_{2}} \cdot(\mathrm{RT})^{\left(m_{1}+m_{2}\right)}}{\mathrm{C}_{\mathrm{A}}^{n_{1}} \times C_{\mathrm{B}}^{n_{2}} \cdot(\mathrm{R} T)^{\left(n_{1}+n_{2}\right)}}\)
= \(\frac{\mathrm{C}_{\mathrm{C}}^{m_{1}} \times \mathrm{C}_{\mathrm{D}}^{m_{2}}}{\mathrm{C}_{\mathrm{A}}^{n_{1}} \times C_{\mathrm{B}}^{n_{2}}} \). (RT)(m1+m2) – (n1+n2) ……………….. (iv)
From eqns. (i) and (iv),
Kp = Kc (RT)∆n
Where, ∆n = (m1+m2) – (n1+2)
= Total number of products – Total number of reactants.
Question 2.
In the equilibrium shown below at 899 K value of Kp is 0.04 atm. What will be the concentration of C2H6 at equilibrium, if at 4.0 atm pressure C2H6 is placed in a flask and allowed to reach equilibrium ? .
C2H6(g) ⇌ C2H4(g) + H2(g)
Solution:
C2H6(g) ⇌ C2H4(g) +H2(g)
![]()
Kp = [Latex]\frac{P_{C_{2}} \mathrm{H}_{4} \cdot P_{\mathrm{H}_{2}}}{\mathrm{P}_{\mathrm{C}_{2} \mathrm{H}_{6}}} [/latex] = \(\frac{P . P}{4 \cdot 0-P}\)
or 0.04=\(\frac{P^{2}}{4 \cdot 0-P}\) or 016.0 – 0.04P= P2
or P = \(\frac{-0-04 \pm \sqrt{0-0016-4(-0 \cdot 16)}}{2}\)
or P = \(\frac{-0.04 \pm 0 \cdot 80}{2}\)
or P = 0.38 (On taking positive value)
Thus, PC2H6 = 4.0 – 0.38 = 3.62 atm.
Question 3.
Calculate the equilibrium constant for the following equations :
(1) PCl5⇌ PCl3 + Cl2,
(2) H2 +I2 ⇌2HI.
Answer:
1. Let a mole of PCl5start the reaction, and at equilibrium x mole dissociate. If volume of the container is v litre, then.
PCl5⇌ PCl3 + Cl2,

According to the law of mass action Kc = \(\frac{\left[\mathrm{PCl}_{3}\right]\left[\mathrm{Cl}_{2}\right]}{\left[\mathrm{PCl}_{5}\right]}\)
On substituting the values,
Kc = \(\frac{\left[\frac{x}{v}\right]\left[\frac{x}{v}\right]}{\left[\frac{a-x}{v}\right]} \) or Kc = \(\frac{x^{2}}{v^{2}}\) × \(\frac{v}{(a-x)}\)
or Kc = \(\frac{x^{2}}{(a-x) v}\)
2. Synthesis of HI : Let n and b mole of H2 and I2 are taken to react initially. At equilibrium x moles of both react. If volume of container is v litre then
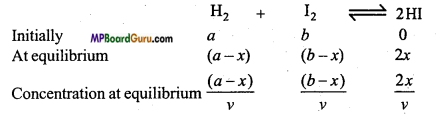
According to law of mass action,
Kc = \(\frac{[\mathrm{HI}]^{2}}{\left[\mathrm{H}_{2}\right]\left[\mathrm{I}_{2}\right]}\)
On substituting the values,
Kc = \(\frac{\left(\frac{2 x}{v}\right)^{2}}{\left(\frac{a-x}{v}\right)\left(\frac{b-x}{v}\right)}\)
or Kc = \(\frac{4 x^{2}}{(a-x)(b-x)}\).
![]()
Question 4.
Derive Ostwald’s dilution law related to the ionization of weak electrolytes. What are its limitations ? Or, Establish relation between extent of ionization and ionization constant.
Answer:
Ostwald’s dilution formula : In 1888 Ostwald stated that: Weak electrolytes are partially ionised. The ions produced due to ionization of weak electrolyte exist in dynamic equilibrium with the undissociated molecules.
On this ionic equilibrium, law of mass action can be applied which is known as Ostwald’s dilution law.
Let AB be a weak electrolyte, whose one mole is dissolved in v litre, if degree of

According to the law of mass action,
K = \(\frac{\left[A^{+}\right]\left[B^{-}\right]}{[\mathrm{AB}]}\)
On substituting the values,
K = \(\frac{\left[\frac{\alpha}{v}\right]\left[\frac{\alpha}{v}\right]}{\left[\frac{1-\alpha}{v}\right]}\)
⇒ K = \(\frac{\alpha^{2}}{v^{2}} \cdot \frac{v}{(1-\alpha)}\)
or K = \(\frac{\alpha^{2}}{v(1-\alpha)}\)
Degree of ionization of weak electrolyte is extremely less. Thus, value of a is negligible.
On substituting (1 – α) = 1,
K = \(\frac{\alpha^{2}}{v}\)
or Kv =α2
or \(\sqrt{\mathrm{K} v}\) = α
or \(\sqrt{\mathrm{K} \frac{1}{c}}\) = α [∵ \(\frac{1}{v}\) = c]
Thus, degree of ionization of weak electrolyte is directly proportional to the square root of its dilution and inversely proportional to square root of its concentration.
Question 5.
Write the relation between ΔG and Q and define the meaning of its step. Also answer the following questions :
(a) When Q < K, why does the reaction proceed towards the forward direction and when Q = K then why does the resultant reaction does not occur?
(b) State the effect of increase in pressure on reaction quotient of the following reaction:
CO(g) + 3H2(g) ⇌ CH4(g) + H2O(g)
Answer:
Relation between ΔG and Q is :
ΔG = ΔG°+ RT In Q
Where ΔG = Change in free energy as a result of the reaction, ΔG° = Standard free energy, Q = Reaction quotient, R = Gas constant, T = Absolute temperature.
(a) ΔG°=-RTlnK
∴ ΔG = -RT In K + RT In Q
or ΔG = RTln\(\frac{\mathrm{Q}}{\mathrm{K}}\)
If Q < K, ΔG will be negative and reaction will proceed in the forward direction.
If Q = K, ΔG = Zero, the reaction will be in equilibrium and there will be no effect on the reaction.
(b)CO(g) + 3H2(g) ⇌ CH4(g) + H2O(g) .
∴ Kc = \(\frac{\left[\mathrm{CH}_{4}\right]\left[\mathrm{H}_{2} \mathrm{O}\right]}{[\mathrm{CO}]\left[\mathrm{H}_{2}\right]^{3}}\)
On decreasing the pressure, volume decreases. On doubling the pressure, volume remains half but molar concentration becomes double, then,
Qc = \(\frac{2\left[\mathrm{CH}_{4}\right] \cdot 2\left[\mathrm{H}_{2} \mathrm{O}\right]}{2[\mathrm{CO}]\left\{2\left[\mathrm{H}_{2}\right]\right\}^{3}}\)= \(\frac{1}{4} \frac{\left[\mathrm{CH}_{4}\right]\left[\mathrm{H}_{2} \mathrm{O}\right]}{[\mathrm{CO}]\left[\mathrm{H}_{2}\right]^{3}}
\) = \(\frac{1}{4}\) Kc
Thus, Q is less than Kc, thus to regain the equilibrium Q will try to increase due to which the reaction will proceed in the forward direction.
Question 6.
What is degree of ionisation? What are factors which influences the ionization?
Ans. Electrovalent compounds dissolve in solvent (water) and provide ions. The part of the total quantity ionised, is commonly known as degree of ionisation. It is denoted by α.
Thus, Degree of ionisation = \(\frac{\text { Number of dissociated molecules }}{\text { Total number of molecules }}\)
Factors which influence ionization:
- Nature of solute: The salt formed by strong acid and strong base are completely ionised while salts formed by weak acid and weak base are partially ionised.
- Nature of solvent: Solvent weakens the attractive force existing between ions. This nature of solvent is known as dielectric constant. The solvent having higher Value of dielectric constant (e.g., Water) is supposed good solvent for ionisation.
- Concentration: Ionisation of any electrolyte is inversely proportional to concentration of solution. Thus less concentration favours more ionisation.
- Temperature: Higher temperature value favours ionisation because of high temperature, the mutual attractive force existing between ions becomes weak.
- Presence of other ions: Presence of other ions in the solution also affects the ionisation. For example, in presence of H+, ionisation of H2S becomes suppressed while in presence of OH–, ionisation of H2S is increased.
Question 7.
What are Acids and Bases? How is their relative strength determined?
Answer:
Acid: According to Arrhenius, acid is a substance which dissolve in water to liberate H+ ion.
HCl ⇌ H+ + Cl–
According to Bronsted-Lowry concept, acid is a substance which donates a proton to another compound or substance in solution.
HCl + H2O ⇌ Cl– + H3O+
According to Lewis concept, an acid a substance which can accept an electron pair from other substance.
H3N: + BF3 → H3N → BF3 Lewis acid
Strength of Acid: Strength of an acid can be represented by its ionisation constant.
HA + H2O ⇌ A– + H3O+
According to the law of mass action,
K = \(\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]\left[\mathrm{H}_{2} \mathrm{O}\right]}\)
or K[H2O] = \(\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right][\mathrm{A}]}{[\mathrm{HA}]}\)
or Ka = \(\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]} \) [ ∵K[H2O] = Ka]
Where Ka is tlie ionisation constant of the acid, which is known as Acidity constant. Higher the value of Ka, higher will be the strength of acid.
Base: According to Arrhenius, base is a substance which dissolves in water to liberate OH– ions.
KOH ⇌ K+ + OH–
According to Bronsted and Lowry, base is a substance which accept a proton from any other substance in solution.
NH3+ H2O ⇌ NH4++OH–
According to Lewis concept, base is a substance which can donate a pair of electrons.
H3N:+ BF3 → H3 N → BF3 Lewis base
Strength of Base: Strength of a base depends on its tendency to accept a proton. Strength of base can be determined by the help of its ionisation constant.
NH3 + H2 O ⇌ NH4 + + OH–
According to the law of mass action :
K=\(\frac{\left[\mathrm{NH}_{4}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{NH}_{3}\right]\left[\mathrm{H}_{2} \mathrm{O}\right]} \)
or K[H2O] = \(\frac{\left[\mathrm{NH}_{4}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{NH}_{3}\right]}\)
or Kb = \(\frac{\left[\mathrm{NH}_{4}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{NH}_{3}\right]}\) ,[∵ K[H2O] =Kb]
Here Kb is the ionisation constant of base which is known as basicity constant. Higher the value of it, higher will be the strength of the base.
![]()
Question 8.
According to the following endothermic reaction, dihydrogen gas is obtained by oxidation :
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g)
(a) Write the expression of Kp for the above reaction.
(b) How will the composition at equilibrium affected of KP and reaction mixture:
(i) Pressure is increased,
(ii) Temperature is increased,
(iii) Catalyst is used.
Solution:
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g)
(a) KP = \(\frac{\mathrm{P}_{\mathrm{CO}} \cdot \mathrm{P}_{\mathrm{H}_{2}}^{3}}{\mathrm{P}_{\mathrm{CH}_{4}} \cdot \mathrm{P}_{\mathrm{H}_{2} \mathrm{O}}} \)
(b)
- Due to increase in pressure, equilibrium will shift in the direction, where pressure is decreased (i.e., No. of moles of gas is less). As a result of increase in pressure KP will remain same.
- Since ∆H = Positive (Endothermic reaction) thus, reaction will occure.by the absorption of heat.
Thus, with the increase in temperature, equilibrium will displace in that direction where heat is absorbed (i.e., forward direction). Due to this, value of KP increases. - No effect, because catalyst produces same effect on the rate, in both the direction.
Question 9.
What is Le-Chatelier’s principle? What will be the effect of increase in concentration, temperature and pressure in the following reactions :
(a) N2 + 3H2 ⇌ 2NH3 ; ∆H =-93.6 kJ
(b) N2 + O 2 ⇌ 2NO; ∆H =+180.7 kJ
Answer:
Le-Chatelier proposed a law, it states “If an equilibrium in a chemical system is disturbed by changing temperature, pressure or concentration of the system, the equilibrium shift in such a way so that the effect of change gets minimised.”
(a) N2 + 3H2 ⇌ 2NH3; ∆H = -93.6 kJ
1. Effect of.pressure : If pressure is increased in the above reaction, then forward reaction will be favoured because on increasing the pressure there is decrease in volume. Thus, that reaction will increase in which volume is decreasing i.e., rate of forward reaction will increase and more of NH3 will be formed.
2. Effect of temperature: Formation of ammonia is an exothermic reaction. On increasing the temperature the reaction proceeds in that direction where heat decreases thus production of NH3 will decrease.
3. Effect of concentration : On increasing the concentration of N2 and H2, formation of NH3 increases because value of \(\frac{\left[\mathrm{NH}_{3}\right]^{2}}{\left[\mathrm{~N}_{2}\right]\left[\mathrm{H}_{2}\right]^{3}} \) should remain constant.
(b) N2+O2 ⇌2NO; ∆H =+180.7 kJ
1. Effect of concentration: On increasing concentration of N2 and O2, more NO will be formed.
2. Effect of pressure: Pressure has no effect because total volume of reactants and products is the same.
![]()
3. Effect of temperature: Formation of NO is an endothermic reaction (because ∆H is +ve), so on increasing the temperature, more NO will be formed.
Question 10.
Write Le-Chatelier’s principle? What are the necessary conditions required for the high production of sulphur trioxide in equilibrium by the help of this law?
2SO2 + O2 ⇌ 2SO3 ; Δ=-188.2kJ
Answer:
Le-Chatelier’s principle: Le-Chatelier proposed a law, it states “If an equilibrium in a chemical system is disturbed by changing temperature, pressure or concentration of the system, the equilibrium shift in such a way so that the effect of change gets minimised.”
(a) N2 + 3H2 ⇌ 2NH3; ∆H = -93.6 kJ
1. Effect of pressure: If pressure is increased in the above reaction, then forward reaction will be favoured because on increasing the pressure there is decrease in volume. Thus, that reaction will increase in which volume is decreasing i.e., rate of forward reaction will increase and more of NH3 will be formed.
2. Effect of temperature: Formation of ammonia is an exothermic reaction. On increasing the temperature the reaction proceeds in that direction where heat decreases thus production of NH3 will decrease.
3. Effect of concentration : On increasing the concentration of N2 and H2, formation of NH3 increases because value of \(\frac{\left[\mathrm{NH}_{3}\right]^{2}}{\left[\mathrm{~N}_{2}\right]\left[\mathrm{H}_{2}\right]^{3}} \) should remain constant.
Question 11.
According to Le-Chatelier’s principle explain the following effects on the following physical equilibria:
(1) Effect of temperature and pressure on melting of ice.
(2) Effect of temperature on the evaporation of water.
(3) Effect of temperature on solubility in water.
Answer:
1. Effect of temperature and pressure on the melting of ice :
Ice , ⇌ Water – Q cal
Conversion of ice to water is an endothermic reaction and volume decreases. Therefore increase in temperature and pressure displaces the equilibrium towards forward direction.
2. Effect of temperature and pressure on Evaporation of water :
Water⇌ Water vapour – Q cal
During the evaporation of water, volume increases. Therefore, on increasing the pressure equilibrium displaces towards backward direction. Thus increase in pressure leads to less evaporation and the process is endothermic. Therefore, increase in pressure displaces the reaction towards forward direction.
3. Effect of temperature on solubility in water: Dissolution of NH4Cl, NaCl in water is an endothermic process. Solubility of such salts in water increases with the increase in temperature. Alternatively solubility of CaCO3 and CaO in water is an exothermic reaction. Therefore, solubility of such salts in water decreases with the increase in temperature.
![]()
Question 12.
Explain chemical equilibrium by taking the example of formation and dissociation of hydrogen iodide.
Answer:
At 720 K reaction between H2 and I2 taking place in a closed container can be expressed as: ,
H2(g) + I2(g) → 2HI(g)
HI is formed by the collisions between H2 and I2 because reaction is taking place in a closed container and molecules cannot come out and collide with each other, thus reaction proceeds in both the directions and it is a reversible reaction.
Forward reaction: H2(g) + I2(g) → 2HI(g)
Backward reaction : 2HI(g) → H2(g) + I2(g)
Reversible reaction: H2(g) + I2(g) → 2HI(g)
Initially, concentration of reactant is high, thus rate of forward reaction is high. Rate of forward reaction decreases with time and concentration of products in backward direction increases, as a result rate of back- ward reaction increases. At a state rate of reaction in both the directions becomes same i.e. dissociation of HI, because equal to formation of HI. This state is known as equilibrium state of reversible reaction.
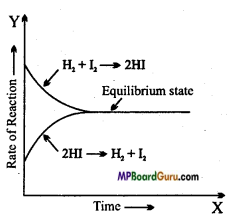
Question 13.
What is buffer solution ? Explain buffer action of acidic buffer.
Answer:
Buffer solution: Buffer solution : Thus, solution in which,
(i) pH value is definite.
(ii) pH is not changed on dilution or on keeping for sometime.
(iii) On adding acid or base in less quantity, pH change is negligible.
Such solutions are called buffer solutions.
or.
Buffer solutions are solutions which retain their pH constant or unaltered.
Buffer action of acidic buffer : Mixture of acetic acid and sodium acetate is an example of acidic buffer. Ionization of sodium acetate will produce acetate ions in large quantity while H ion will be less because of weak acid and common ion effect.
CH3COONa CH3COONa ⇌ CH3COO– +Na+
CH3COOH ⇌ CH3COO– + H+
On adding HCl, H+ ions produced will combine with acetate ions to form weakly ionized CH3COOH, so its pH will not change.
H+ + CH3COO– ⇌ CH3COOH Weak acid
On adding a drop of NaOH, OH– ion are produced which combine with H+ of acetic acid to produce weakly ionized H2O. Thus, pH remains constant.
OH– + CH3COOH→ H2O + CH3COO–.
NaOH ⇌ Na+ + OH–
OH– + H+ ⇌ H2O
Question 14.
Explain buffer action of basic buffer.
Answer:
Buffer action of basic buffer: Mixture of NH4OH and NH4Cl is an example of basic buffer solution. NH4Cl is a strong electrolyte which suppresses the ionization of weak electrolyte NH4OH due to common ion [NH4+], As a result the number of [OH–] ion decreases.
NH4 Cl ⇌ NH4++Cl–
NH4OH ⇌ NH4+ +OH–
Suppose a drop of NaOH is added to this buffer, then the OH– ion of NaOH combines with the NH4+ ion of NH4Cl and form NH4OH. Since NH4OH is a weak electrolyte it dissociates very feebly hence the number of OH- ion does not increase.
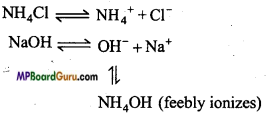
On adding an acid H+ ion obtained combines with OH– to form H2O. In this condition,
to maintain equilibrium some NH4OH is dissociated and this way OH–ion concentration remain constant and pH value remains unchanged.
HCl ⇌ H+ + Cl–
H+ + OH– ⇌ H2O
Importance of Buffer Solution :
Uses:
1. In laboratories: In study of velocity of chemical reactions, buffers are used.
2. Qualitative analysis: In removal of phosphate ion buffer of CH3COONa and CH3COOH is used.
3. Industries: In production of alcohol by fermentation, buffers are used (pH between 5 to 6-8). In manufacturing of sugar, paper and in electroplating industries, buffers are used.
Question 15.
Establish the relation in pH and pOH value. Or, Prove that pH + pOH = 14.
Answer:
By self ionisation of water
H2O + H2O ⇌ H3O+ + OH–
According to the law of mass action,
K = \(\frac{\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{H}_{2} \mathrm{O}\right]^{2}}\)
or K[H2O]2=[H3O+][OH–], [ ∵K[H2O]2 =Kw]
or Kw, = [H3O+][OH–] ………………………. (i)
At 298 K, Kw = 1 × 10-14, it’s a constant known as ionic product of water.
On substituting the value in eqn. (i),
10-14 =[H3O+][OH–]
On taking log10 on both side,
-14log1010 = log10[H3O+] + log10[OH–]
or -14 = log10[H3O+] + log10[OH–] [∵ log1010=l]
Multiplying both side by (-),
or 14 = [-log10[H3O+]+[-log10[OH–]]
or 14 = pH + pOH, [-log10[H3O+] = pH,-log10[OH–] = pOH].
Question 16.
Derive Henderson equation to calculate the pH value of Buffer solution.
Answer:
A weak acid HA and its ionized salt NaA is taken.
HA ⇌ H+ + A+
Ka = \(\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}\)
or [H+] = Ka \(\frac{\text { [HA] }}{\left[\mathrm{A}^{-}\right]}\)
Suppose molecular concentration of acid and base in the mixture is C1 and C2. Due to the presence of A– ion in the salt, dissociation of acid in solution decreases.
[HA] = C1
[A– ] = C2
[H+] = Ka \(\frac{\mathrm{C}_{1}}{\mathrm{C}_{2}}\)
or [H+] = Ka \(\frac{\text { [Acid] }}{\text { [Salt] }}\)
On taking log10 both sides log 10[H+] = log 10 Ka + log 10\(\frac{\text { [Acid] }}{\text { [Salt] }}\)
⇒ – log10 [H+] = – log10 Ka – log10\(\frac{\text { [Acid] }}{\text { [Salt] }}\)
⇒ – log 10 [H+] = – log10 Ka + log10\(\frac{\text { [Acid] }}{\text { [Salt] }}\)
⇒ pH = pKa + log10\(\frac{\text { [Acid] }}{\text { [Salt] }}\)
This is Henderson’s reaction.
Question 17.
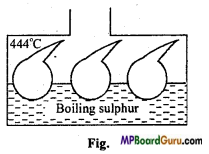
Explain an experiment to prove practically the law of mass action.
Answer:
In a few bulbs of glass, different amounts of H2 and I2 is taken and their mouth are closed. These bulbs are heated on sulphur vapours for some time due to which equilibrium is established very soon. These bulbs are suddenly cooled to room temperature to establish equilibrium. On opening these bulbs in NaOH solution, NaOH solution absorbs HI and iodine of each bulb. Volume of the remaining hydrogen is determined and concentration of HI and I2 at equilibrium is also determined. This way amount of H2,I2 and HI in each bulb is determined and by their value of K for each bulb is calculated.
If value of K of each bulb is same, it justifies the law of mass action.
Question 18.
At 1127K and 1 atm pressure in gaseous mixture of CO and CO2 at equilibrium in solid carbon 90.55% CO is present.
C(s) + CO2(g) ⇌ 2CO(g)
Calculate the value of K for the reaction at the above temperature.
Solution:
Reaction C(s) + CO2(g) ⇌ 2CO(g)
If total mass of CO and CO2 mixture is, 100gm, then
CO 90.55 gm and CO2 = 100- 90.55 = 9.45gm
No. of moles of CO=\(\frac{90 \cdot 55}{28} \) =3234
No.of molesof CO2 =\(\frac{9 \cdot 45}{44}\) =0.215
Pco = \(\frac{3.234}{3 \cdot 234+0 \cdot 215}\) × 1 atm = 0.938 atm
Pco2 = \(\frac{0 \cdot 215}{3 \cdot 234+0 \cdot 215}\) × 1 atm = 0.062 atm
Kp = \(\frac{\mathrm{P}^{2} \mathrm{cO}}{\mathrm{P}_{\mathrm{CO}_{2}}}\) = \(\frac{(0 \cdot 938)^{2}}{0 \cdot 062}\) = 14.19
Now, Δn(g) = 2 – 1 = 1
Kp = Kc × RTΔn(g)
R = 0.0821 L atm K-1 mol-1, T = 1127 K
Kc = \(\frac{\mathrm{K}_{p}}{\mathrm{RT}}\) = \(\frac{14 \cdot 19}{0 \cdot 0821 \times 1127} \) = 0.153.
![]()
Equilibrium Class 11 Important Numerical Questions
Question 1.
At 293 K solubility product of AgCl in water is 1.5 × 10-10, calculate its solubility at this temperature. (Molecular mass of AgCI = 143.5) (NCERT)
Solution:
Solubility = \(\sqrt{\text { Solubility product }}\)
S = \(\sqrt{\mathrm{K}_{s p}}\)
= \(\sqrt{1 \cdot 5 \times 10^{-10}}\)
= 1.22 × 10-5 gm mol per litre
= 1.22 × 143.5 × 10-5 gm per litre
= 1.75 × 10-3 gm per litre
Question 2.
At 700 K temperature for 2SO3(g) ⇌ 2SO2(g) + O2(g) ; Kp = 1.8 × 10-3. Determine the value of Kc in mol per litre at this temperature. (NCERT)
Solution:
Kp = Kc × RT Δn
or Kc = \(\frac{\mathrm{K}_{p}}{\mathrm{RT}^{\Delta n}}\)
Given T = 700K
R = 8.31Jmol-1 K-1,
Kp = 1.8 × 10-3 kPa = 1.8 pa
On subsututing the values,
Kc = \(\frac{1 \cdot 8}{8 \cdot 31 \times 700}\)
= 3.09× 10-4 mol L-1.
Question 3.
At 444°C, 15 gm mol of H2 and 5.2 gm mol of I2 react to form 10 gm mole HI. Calculate the equilibrium constant for the reaction, (NCERT)
H2 + I2 ⇌ 2HI.
Solution:
H2 + I2 ⇌ 2HI.
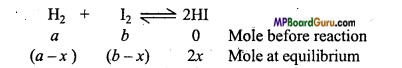
K = \(\frac{4 x^{2}}{(a-x)(b-x)} \)
Given, a = 15, b = 5.2, 2x = 10 or x = 5.
∴Kc = \(\frac{4 \times(5)^{2}}{(15-5)(5 \cdot 2-5)}\)
= \(\frac{4 \times 25}{10 \times 0 \cdot 2}\) = 50.
Question 4.
At atmospheric pressure and 250°C temperature 80% PCl5 dissociates. Determine the value of equilibrium constant Kp. (NCERT)
Solution:
For the dissociation of PCl5, the reaction is:
PCl5 ⇌ PCl3 + Cl2
![]()
x = 80% i.e. \(\frac{80}{100}\) = 0.8 mole.
Total No. of molecules at equilibrium = 1- x + x + x
= 1-0.8 + 0.8 +0.8
= 1.8
Ppcl5 = \(\frac{0 \cdot 2}{1 \cdot 8}\) × 1,
Ppcl3 = \(\frac{0 \cdot 8}{1 \cdot 8} \) × 1,
Ppcl2 = \(\frac{0 \cdot 8}{1 \cdot 8} \) × 1
∴ Kp = \(\frac{\frac{0 \cdot 8}{1 \cdot 8} \times \frac{0 \cdot 8}{1 \cdot 8}}{\frac{0 \cdot 2}{1 \cdot 8}} \)
or Kp = \(\frac{0 \cdot 8}{1 \cdot 8}\) × \(\frac{0 \cdot 8}{1 \cdot 8} \)×\(\frac{0 \cdot 2}{1 \cdot 8}\) = 1.78
Question 5.
Determine the pH of 0.0001M NaOH solution. Sol. In 0.0001M NaOH solution
Solution:
In 0.0001M NaOH solution
[OH–] = 10-4
or [H+] = \(\frac{10^{-14}}{10^{-4}}\) = 10-10
By the definition of pH [H+] = 10-H+
Thus, pH of solution pH = 10
Alternative method :
pH = – log 10 [H+]
pH = – log 10 [10-10 ] = 10 log10 10, [∵ log10 10 = 1]
Question 6.
At 298 K, NOM2 is formed by NO and O2.
(a) NO(g) +O2(g) ⇌ NO2(g)
Calculate (a) ΔG° and
(b) equilibrium constant for the reaction.
ΔfG°(NO2) = 52.0kJ/mol, Δf G°(NO) = 87 .0 kJ/mol and Δf G°(O2)
Solution:
(a) Reaction NO(g) +\(\frac{1}{2}\)O2(g) ⇌ NO2(g)
For the reaction, ΔG° = ΔG°f(NO2) – ΔG°f(O2)
= (52.0 – 87.0)KJ/mol = – 35.0 KJ / mol
(b) Kc = – ΔG° = 2.303 RTlog Kc
-(-35.0) = 2.303 × 0.008314 × 298 log Kc
35.0 = 5.7058 log Kc
log Kc = \(\frac{35}{5 \cdot 7058}\) = 6.134
Kc = anti log 6.314
Kc = 1.36 × 106
![]()
Question 7.
Solubility of PbSO4 in water at 25°C is 0.038 gm/litre. Determine solubility product at this temperature. (NCERT)
[Pb = 207,S = 32,O = 16]
Solution:
Molecular mass of PbSO4 = 303
Solubility of PbSO4 = \(\frac{0.038}{303}\) gm mol per litre
or Solubility product = (Solubility)2
= \(\left(\frac{0.038}{303}\right)^{2}\) = 1.57 × 10 -8
Question 8.
If at 298 K temperature, ionisation of 0.1M CH3 COOH solution is 1.33%, then calculate its ionisation constant. (NCERT)
Solution:
% ionisation of CH3COOH is 1.33, thus α = 0.0133
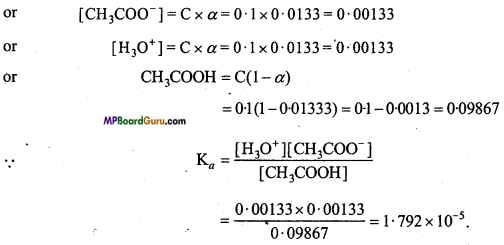
Question 9.
pH of 0.005M Codiene (C18H21NO3) is 9.95. Determine its ionization constant. (NCERT)
Solution:
Molar concentration of Codiene = 0.005 M
Codiene + H2O ⇌ Codiene H+ + OH–
pH = 9.95
pOH = 14 – 9.95 = 4.05
or -log[OH–] = 4-05 or log [OH–] = -4.05
or [OH–] = Anti log [-4.05] = 8.913 × 10-5M
Kb = \(\frac{\text { Codiene }\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]}{\text {Codiene }} \) = \(\frac{\left[\mathrm{OH}^{-}\right]^{2}}{\text { Codiene }}\)
∵ [OH–] = \(\sqrt{\mathrm{K}_{b} \times \mathrm{C}}\)
or 8.913 × 10-5 = \(\sqrt{\mathrm{K}_{b} \times 0.005}\)
∴ Kb = 1.58 × 10-6.
Question 10.
Determine the pH of the following resultant mixture :
(a) 10 ml of 0.2 M Ca(OH)2 + 25 ml of 01 M HCl
(b) 10 ml of 01M Ca(OH)2 + 10 ml of 0 01 M H2SO4
(c) 10 ml of 01M H2SO4 + 10 ml of 0.01 M KOH.
Solution:
(a) Base Ca(OH)2 = Acid HCl
M1V1 = M2V2
According to the question,
M1 = [OH– ] = 2 × 0.2 = 0.4M
V1 =10 ml
M2 = 0.1M ,
V2 = 25 ML
M1V1 = 0.4 × 10= 4
M2V2 =0.1 × 25 = 2.5 .
4>2.5
M1V1 > M2V2 .
Thus, the mixture is basic.
pH >7
Thus, [OH–] = \(\frac{\mathrm{M}_{1} \mathrm{~V}_{1}-\mathrm{M}_{2} \mathrm{~V}_{2}}{\mathrm{~V}_{1}+\mathrm{V}_{2}} \)
= \(\frac{4-2 \cdot 5}{10+25}\) = 0.043 M
pOH = -log[4.3 × 10-2] = 2 – 0.6335 = 1.3665
pH = 14-1.3665 = 12.6335 ≈ 12.63.
(b) Similarly, Base Ca(OH)2 = Acid H2SO4
M1 V1 = M2V2
M1V1 =(2 × 0.01) × 10 = 0.2
M2V2 =(2 × 0.1) × 10 =2
∵ 0.2 < 2
∴M1 V1 < M2V2. Thus, mixture is acidic.
pH < 7.
(c) Acid H2SO4 = Base KOH
M1V1 =M2V2
M1V1 = (2 × 0.1) × 10= 2
M2V2 =0.01 × 10 = 0.1
M1V1 >M2V2.
Thus, mixture is acidic.
[H+] = \(\frac{\mathrm{M}_{1} \mathrm{~V}_{1}-\mathrm{M}_{2} \mathrm{~V}_{2}}{\mathrm{~V}_{1}+\mathrm{V}_{2}}\) = \(\frac{(0 \cdot 2 \times 10)-(0 \cdot 01 \times 10)}{10+10}\) = \(\frac{2-0 \cdot 1}{20}\)= 0.09 M
pH = – log[0.09] = – log [9.0 × 10 -2]
pH = 2 – 0.09542 = 1.045 ≈ 1.05
Question 11.
The concentration of sulphide ion in 0.1M HC1 solution saturated with hydrogen sulphide is 1.0 × 10-19 M. If 10 ml of this is added to 5 ml of 0.04 M solution of the following: FeSO4, MnCl2, ZnCl2 and CdCl2 in which of these solutions precipitation will take place ?
Ksp (FeS) = 6.3 × 10-18, Ksp (MnS) = 2.5 ×10-13,
Ksp (ZnS) = 1.6 × 10-24, Ksp (CdS) = 8.0 × 10-27.
Solution:
For precipitation, Ionic product > Solubility product.
Ionic product of each salt is calculated and compared with solubility product.
[S2-] = 1 × 10-19 M
10 ml S2- solution is added 5 ml of 0.04 M of different solutions.
Thus, final volume of the solution will be 10 + 5 = 15 ml
[S2-] =\(\frac{10 \times 10^{-19}}{15}\) = 6.67 × 10-20M
Where [M2+] = Fe+2,Mn+2,Zn+2,Cd2+
[M2+][S2-] = 1.33 × 10-2 × 6.67 × 10-20
Thus, ionic product = 8.8 7 ×10.22
∵ Ionic product of [M2+][S2-] > Ksp of ZnS and CdS.
Thus, these (CdCl2 and ZnCl2) salts will-precipitate in the form of CdS and ZnS.
Question 12.
Determine the solubilities of Silver chromate, Barium chromate, Ferric hydroxide, Lead chloride and Mercurous iodide at 298 K from their solubility product constants. Also determine the molarities of individual ions.
Ksp(Ag2CrO4) = 1.1 × 10-12
Ksp(BaCrO4) = 1.2 × 10 -10
Ksp(Fe(OH)3) = 1.0 × 10-38
Ksp (PbCl2) = 1.6 × 10-5
Ksp (Hg2I2) = 4.5 × 10-29
Solution:
(a) 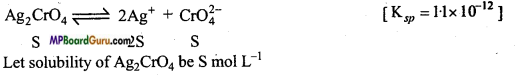
∵ Ksp = [Ag+]2 .[CrO4-2]
∴ Ksp = [2S]2.[S] = 4S3
S3 = \(\frac{\mathrm{K}_{s p}}{4}\) = \(\frac{1 \cdot 1 \times 10^{-12}}{4}\) = 0.275× 10-12
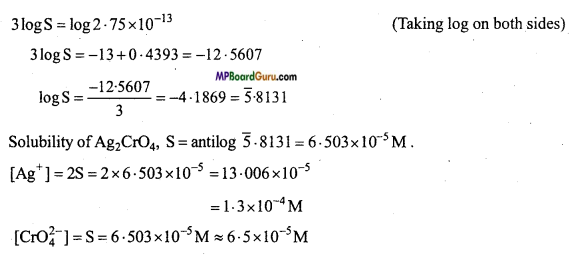
(b) 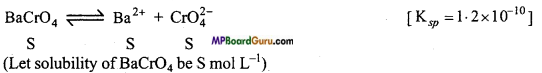
∵ Ksp = [Ba2+] [CrO-24]
Ksp = 1.2 × 10-10 = [Ba+] [CrO-24]
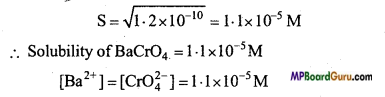
(c) 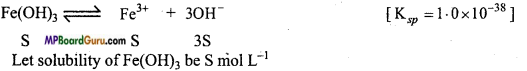
Ksp = [Fe3+][OH–]3
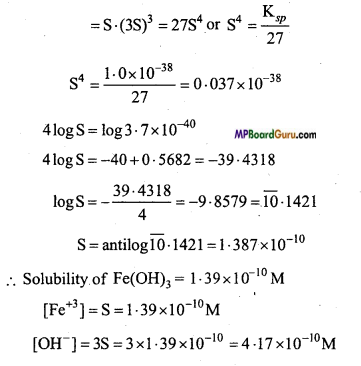
(d)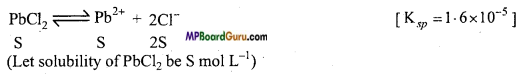
Ksp = [Pb2+] [Cl–]2
Ksp = S.(2S)2 = 4S3
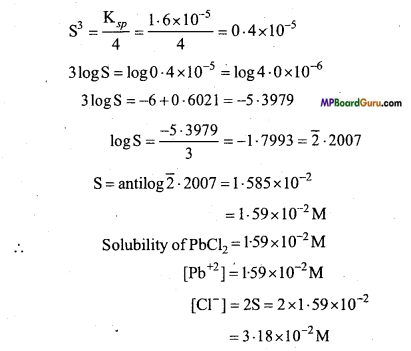
(e) 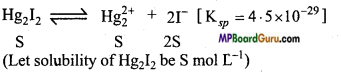
Ksp = [Hg22+][I–]2
Ksp = S .(2S)2 = 4S3
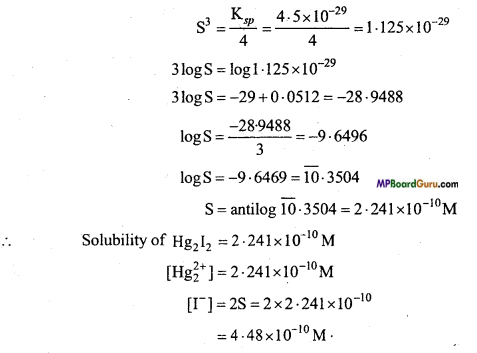
Question 13.
Calculate the pH value of the solution obtained on mixing equal volume of two strong acids A and B of pH 6 and 4.
Solution:
∵ pH of A = 6
∴ [H+] = 10-6mol L-1
∴ pH of B = 4
∴ [H+] = 10-4 mol L-1
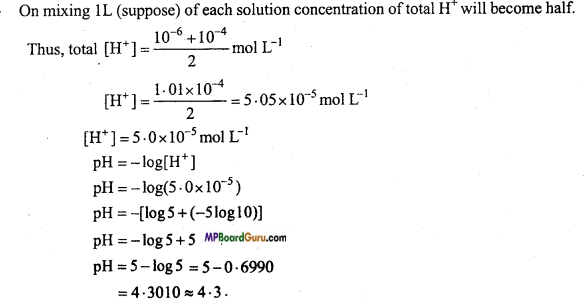
Question 14.
pH of a strong acid Is 50. What will be the pH of the solution obtained by diluting the above solution to 100 time%?
Solution:
Given pH = 5
∴ [H+] = 10-5 mol L-1
.On diluting the solution to 100 times [H+] =\(\frac{10^{-5}}{100}\) = 10-7mol L-1
Total H+ ion concentration = (H+ obtained from acid ) + (H+ obtained from water)
[H+] = 10-7 + 10-7 = 2 × 10-7M
pH = – log [2 × 10-7]
pH = 7 -0.3010 = 6.699.
Question 15.
Ionisation constant of Benzoic add is 6.46 × 10-5 and Ksp of Silver benzoate is 2.5 × 10-13. How many times is Silver benzoate more soluble in a buffer of pH 3.19 compared to its solubility in pure water ?
Solution:
Solubility of Benzoic acid and Silver benzoate
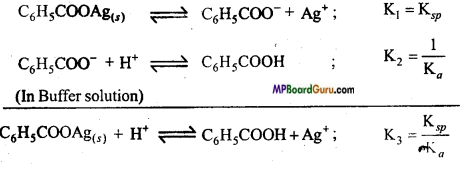
K3 = \(\frac{\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COOH}\right]\left[\mathrm{Ag}^{+}\right]}{\left[\mathrm{H}^{+}\right]} \) = \(\frac{\mathrm{S} . \mathrm{S}}{\left[\mathrm{H}^{+}\right]}\) = \(\frac{\mathrm{K}_{s p}}{\mathrm{~K}_{a}}\)
Where, Solubility of S, C6H5COOAg is S2 = \(\frac{\mathrm{K}_{s p} \times\left[\mathrm{H}^{+}\right]}{\mathrm{K}_{a}} \) ……………………. (i)
In buffer solution of pH = 3.19
log[H+] = -3.19 = \(\overline{4} \cdot 81\)
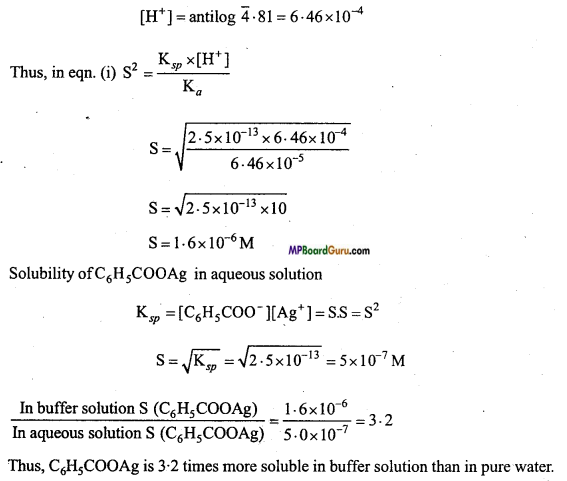
![]()
Question 16.
Ionisation constant of CH3COONa in a buffer solution containing 0.1 mole acetic acid and 0.15 mole sodium acetate is 1.75 × 10-5. Determine the pH of this buffer solution.
Solution:
Henderson equation:
pH = pKa + log \(\begin{array}{l}
\text { [Salt] } \\
\hline \text { [Acid] }
\end{array}\)
= -logl.75 x 10-5+log \(\frac{0 \cdot 15}{0 \cdot 10}\)
= -(-5 log 1.75) + log 1.5
= 4.9
Question 17.
The pH of 0.1 M solution of Cyanic acid (HCNO) is 2.34. Calculate the ionisation constant of the acid and its degree of ionization in the solution.
Solition:
pH of solution = 2.34
HCNO ⇌ H+ + CNO–
-log[H+] = 2.34
[H+] = Anti log \(\overline{3} \cdot 66\) = 4.571 × 10-3 M
[H+] = \(\sqrt{\mathrm{K}_{a} \mathrm{C}}\) = 4.571 × 10-3
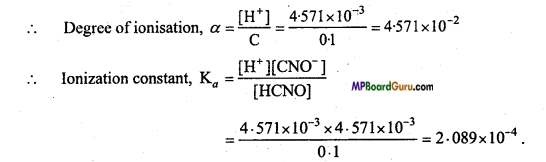
Equilibrium Class 11 Important Questions Objective Type
1. Choose the correct answer:
Question 1.
Which of the following reactions have equal Kc and Kp :
(a) N2(g) + 3H2(g) = 2NH3(g)
(b) 2H2S(g) + 3O2(g) = 2SO2(g) + 2H2O(g)
(c) Br2(g) + Cl2(g) = 2BrCl(g)
(d) P4(g) + 6Cl2(g) = 4PCl3(g).
Answer:
(c) Br2(g) + Cl2(g) = 2BrCl(g)
Question 2.
For the reaction N2(g)+3H2(g) ⇌ 2NH3(g) , ΔH = 92 kJ, the concentration of NH3 at the equilibrium by increase of temperature :
(a) Increases
(b) No change
(c) Decreases
(d) None of these.
Answer:
(c) Decreases
Question 3.
In one litre container equilibrium mixture of reaction 2H2S(g) ⇌ S2(g) is filled. 0.5 mole H2S, 0.1 moIe H2 and 0.4 mole S2 are present in it. Equilibrium constant of this reaction will be :
(a) 0.004 mol litre-1
(b) 0.080 mol litre-1
(c) 0.016 mol litre-1
(d) 0.160 mol litre-1.
Answer:
(c) 0.016 mol litre-1
Question 4.
Favourable conditions for exothermic reaction of ammonia synthesis N2(g)+ 3H2(g) ≅ 2NH3(g) are :
(a) High temperature and high pressure
(b) High temperature and low pressure
(c) Low temperature and high pressure
(d) Low temperature and low pressure.
Answer:
(c) Low temperature and high pressure
Question 5.
Oxidation of SO2 by O2 in SO3 is an exothermic reaction. Manufacture of SO3 will be maximum if:
(a) Temperature is increased and pressure is decreased
(b) Temperature is decreased and pressure is increased
(c) Temperature and pressure both are increased
(d) Temperature and pressure both are decreased.
Answer:
(b) Temperature is decreased and pressure is increased
Question 6.
At 440°C HI was heated in a closed vessel till equilibrium is attained. It is dissociated 22%. Equilibrium constant of dissociation will be :
(a) 0.282
(b) 0.0796
(c) 0.0199
(d) 1.99.
Answer:
(c) 0.0199
Question 7.
Kp and Kc can be expressed as :
(a) Kc = Kp(RT)Δn
(b) Kp = Kc (RT).QΔn
(c) Kp = Kc(RT)Δn
(d) Kc = Kp(RT)Δn.
Answer:
(c) Kp = Kc(RT)Δn
Question 8.
The equilibrium constant of the reaction H2(g)+ I2(g) = 2HI(g) is 64. If the volume of the container is reduced to one fourth of its original volume, the value of the equilibrium constant will be :
(a) 16
(b) 32
(c) 64
(d) 128.
Answer:
(c) 64
![]()
Question 9.
What would happen to a reversible reaction at equilibrium when an inert gas is added while the pressure remains unchanged :
(a) More of the product will be formed
(b) Less of the product will be formed
(c) More of the reactants will be formed
(d) It remains unaffected.
Answer:
(d) It remains unaffected.
Question 10.
Which reaction is not affected by change in pressure :
(a) N2(g) + O2(g) ⇌ 2NO(g)
(b) 2O3(g) ⇌ 3O2(g)
(c) 2NO2(g) ⇌ N2O(g)
(d) 2SO2(g) + O2(g) ⇌ 2SO3(g).
Answer:
(a) N2(g) + O2(g) ⇌ 2NO(g)
Question 11.
SO2(g) + \(\frac{1}{2}\) O2(g) ⇌ SO3(g),
K1 2SO3(g) ⇌ 2SO2(g)+ O2(g), K2
which of the following is correct:
(a) K2 = K1(2)
(b) K2 = K1-2
(c) K2 =K1
(d) K2 =K1-1.
Answer:
(b) K2 = K1-2
Question 12.
For N2 + 3H2 ⇌ 2NH3 + Heat:
(a) Kp = Kc
(b) Kp = Kc RT
(c) Kp = Kc (RT)-2
(d) Kp = Kc (RT)-1.
Answer:
(c) Kp = Kc (RT)-2
Question 13.
Sodium sulphate dissolve in water with the release of heat. Imagine a saturated solution of sodium sulphate. If temperature is increased, then according to Le-Chatelier’s principle:
(a) More of solid will dissolve
(b) Some solid will precipitate in solution
(c) Solution will be more saturated
(d) Concentration of solution will be unchangeable.
Answer:
(b) Some solid will precipitate in solution
Question 14.
For reaction PCl3(g) + Cl2(g) ⇌ PCl5(g) value of Kc at 250°C is 26. Value of Kp at this temperature will be :
(a) 0.61
(b) 0.57
(c) 0.83
(d) 0.46.
Answer:
(a) 0.61
Question 15.
According to Le-Chatelier principle, when heat is given in solid-liquid equilibrium then:
(a) Amount of solid decreases
(b) Amount of liquid decrease
(c) Temperature increase
(d) Temperature decrease.
Answer:
(a) Amount of solid decreases
Question 16.
A chemical reaction is in equilibrium when :
(a) The reactants are completely converted to the products
(b) The rate of forward reaction is equal to the rate of backward reaction
(c) Formation of products is minimum
(d) Reactants and products are equal in quantity.
Answer:
(b) The rate of forward reaction is equal to the rate of backward reaction
Question 17.
At equilibrium, free energy change for reversible reaction will be ;
(a) 0
(b) > 0
(c) ∞
(d) 1.
Answer:
(a) 0
Question 18.
Concentration of reactant remains unchanged in the presence of:
(a) Catalyst
(b) Pressure
(c) Temperature
(d) Concentration.
Answer:
(a) Catalyst
![]()
Question 19.
Water ≅ Vapour, for physical equilibrium, on applying suitable pressure :
(a) Boiling point will increase
(b) Melting point will decrease
(c) Boiling point will decrease
(d) None of these.
Answer:
(a) Boiling point will increase
Question 20.
Number of gram molecules of a substance present in unit volume neutralize :
(a) Activity
(b) Normal solution
(c) Molar solution
(d) Active mass.
Answer:
(c) Molar solution
Question 21.
Whose pH value is maximum :
(a) CH3COOK
(b) Na2CO3
(c) NH4Cl
(d) NaNO3.
Answer:
(b) Na2CO3
Question 22.
pH of 10-8M HCI will be:
(a) 8
(b) 7 .
(c) In between 7 and 8
(d) In between 6 and 7.
Answer:
(d) In between 6 and 7.
Question 23.
If K for N2 + 3H2 ⇌ 2NH3, then K’ for 2N2 + 6H ⇌ 4NH3 will be :
(a) K2
(b) \(\sqrt{\mathrm{K}}\)
(c) \(\frac{1}{\sqrt{K}}\)
(d) \(\frac{1}{\mathrm{~K}^{2}}\) .
Answer:
(a) K2
Question 24.
Ksp value for HgS, Ag2S and PbS is 10-31,10-45 and 10-50. Its solubility order will be :
(a) Ag2S > HgS > PbS
(b) HgS > Ag2S> PbS
(c) HgS < PbS < Ag2S
(d) PbS> Ag2S > HgS.
Answer:
(a) Ag2S > HgS > PbS
Question 25.
Aluminium chloride is:
(a) Bronsted acid
(b) Arthenius acid
(c) Lewis acid
(d) Lewis base.
Answer:
(c) Lewis acid
2. Fill in the blanks:
1. For an endothermic process PCl5 ⇌ PCl3 + Cl2, ΔH = +kcal. Then ………………. temperature and ………………. pressure should be kept (if the process is to take place in forward direction).
Answer:
High, low
2. Ice ⇌ Water – cal. In this reaction high temperature will favour the reaction in direction ………………. and increase in pressure will favour the reaction in ………………. direction.
Answer:
Forward, forward
3. According to Ostwald dilution law, the mathematical expression relating amount of volume and ionization constant is represented as ………………. Degree of dissociation of weak electrolyte is inversely proportional to the ………………. .
Answer:
a = \(\sqrt{\mathrm{VK}_{a}}\) , square root of concentration
4. For reaction AB ⇌ A+ +B– relation between solubility and solubility product can be represented as ………………. .
Answer:
Ksp = [A+][B–]
5. 2SO2 + O2 → 2SO3 + Q cal. In this reaction, for maximum yield of SO3, conditions of temperature and pressure will be ………………. .
Answer:
Low temperature, high pressure
6. Mixture of acetic acid and sodium acetate solution is an example of ………………… .
Answer:
Acidic buffer
7. Mixed solution of ammonium chloride and ammonium hydroxide is an example of ………………… solution.
Answer:
Basic buffer
8. For precipitation, ionic product should be …………………than solubility product.
Answer:
More
9. Ostwald’s dilution law is not applicable for ………………… .
Answer:
Strong electrolyte
10. Effect of temperature on equilibrium constant can be represented by ………………… equation.
Answer:
Van’t H off equation
11. For the reaction N2 + 3H2 ⇌ 2NH3, unit of Kc is …………….. .
Answer:
(mol/litre)-2
12. Value of Kp and Kc change with ……………… .
Answer:
Temperature
13. Higher value of equilibrium constant displaces the reaction move towards ……………… .
Answer:
Forward direction
14. Nature of chemical equilibrium is ……………… .
Answer:
Dynamic.
![]()
3. Match the following:
| ‘A’ | ‘B’ |
| 1. Aqueous solution of KCN | (a) Acidic |
| 2. Aqueous solution of FeCl3 | (b) Neutral |
| 3. Aqueous solution of CH3COONH4 | (c) Basic |
| 4. Aqueous solution of NaCl | (d) Weak acid and base |
| 5. N2 + O2 ⇌ 2NO | (e) K > K |
| 6. PCl5 ⇌ PCl3 + Cl2 | (f) K=K. |
| 7. N2 + 3H2 ⇌ 2NH3 | (g) K< K. |
Answer:
1. (c) Basic
2. (a) Acidic
3. (d) Weak acid and base
4. (b) Neutral
5. (f) K=K.
6. (e) K > K
7. (g) K< K.
4. Answer in one word/sentence:
1. For the unit of Kc concentration is expressed as.
Answer:
mol/litre
2. Manufacture of nitrogen peroxide is an endothermic reaction. What will be the conditions of. temperature and pressure for its maximum yield ?
Answer:
Low temperature and high pressure
3. Ammonia gas dissolve in NH4OH. Here, how does water behave ?
Answer:
Like an acid
4. When NH4Cl is added to NH4OH solution, then the ionisation of NH4OH decreases. What is the reason?
Answer:
Common ion effect
5. At 25°C pH of water is 7, if water is heated to 50°C, what will be the change in pH?
Answer:
pH value will decrease
6. Write the conjugate base of H2PO4- and HCO3-.
Answer:
HPO4-,CO3-
7. Write a substance which can behave both as Bronsted acid and base.
Answer:
HCO3
8. Give an example of basic buffer.
Answer:
NH4OH + NH4Cl
9. Give an example of a salt made lip of weak acid and weak base.
Answer:
Ammonium acetate
10. What is the pH value of human blood?
Answer:
7.4
11. What happens when HCl gas is passed in NaCl solution?
Answer:
NaCl will be precipitated
12. What will be the pH value of 0.1M HCl?
Answer:
1
13. Of the following conjugate bases which is strong: CN– or F–.
Answer:
CN– is strong base.