These MP Board Class 11th Biology Notes for Chapter 10 Cell Cycle and Cell Division help students to get a brief overview of all the concepts.
MP Board Class 11th Biology Notes Chapter 10 Cell Cycle and Cell Division
→ Multicellular organism grows and develop by mitosis cell division, whereas some unicellular organisms reproduce by this division.
→ Body of some lower category of organisms are made up of haploid cells. Zygote of these organ-isms divides by meiosis division to form haploid individual.
→ Meiosis cell division helps in the maintenance of chromosome number in the species.
→ Spindles regulate movement of chromosomes during cell division.
→ Mitosis help to maintain genetic stability.
![]()
→ Nerve cells never divide.
→ Generally in normal condition cells complete cell cycle in 20-25 hours.
→ Duration of cell cycle depends upon type of cell and some external factors such as temperature, nutrition, availability of oxygen, etc.
→ Actual cell division occurs in M-phase of the cell cycle.
→ Duration of cell cycle in bacteria is 20 minutes, in epithelial cell is 8-10 hours and in root cells of onion is 20 hours.
→ Cell division is the basis of continuity of life.
![]()
→ The main difference between the cell division of plant cell and animal cell are in centriole mechanism and cytokinesis process.
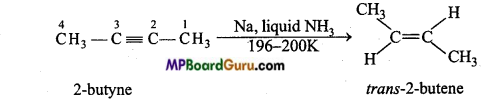
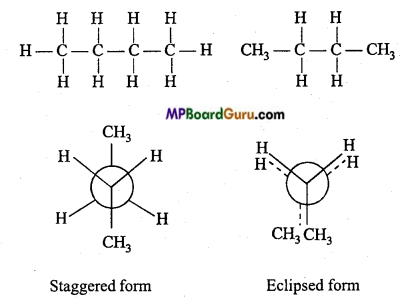
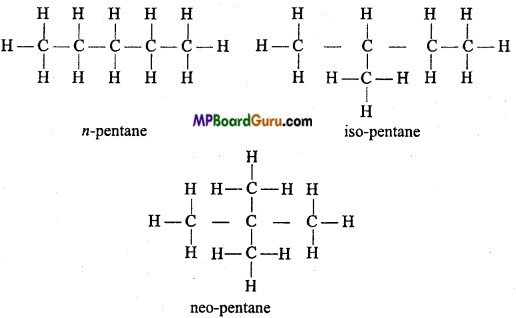
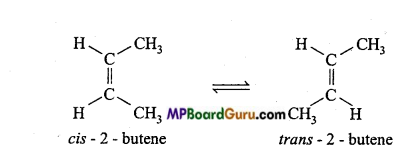
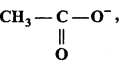
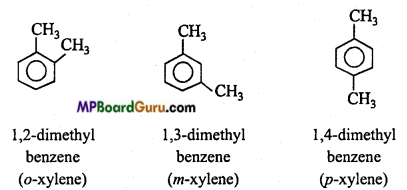
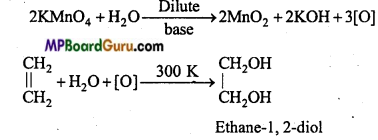
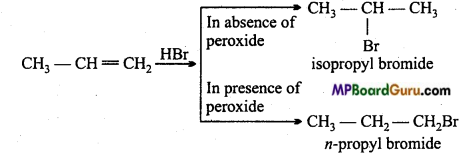
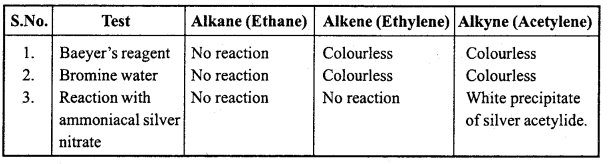



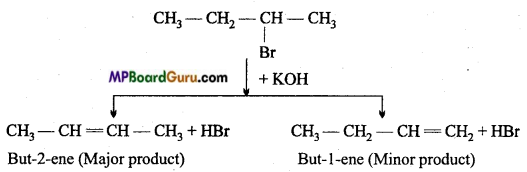
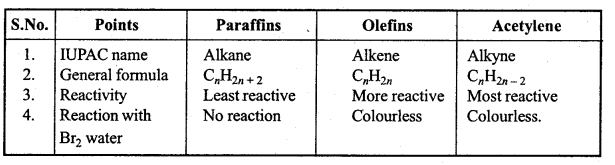
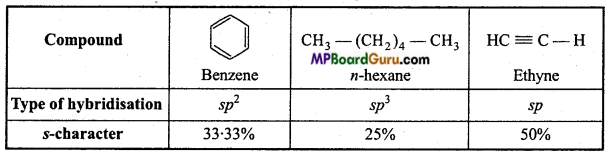
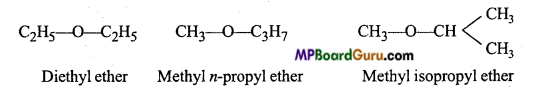


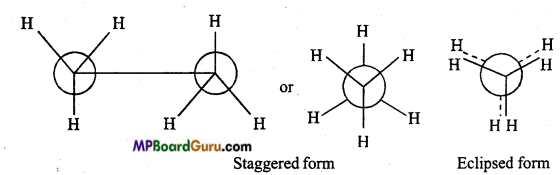
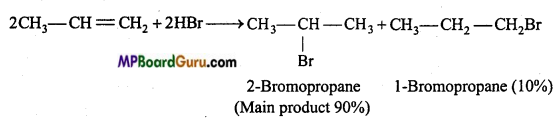
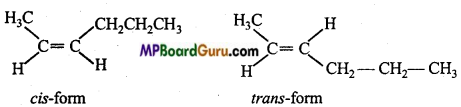
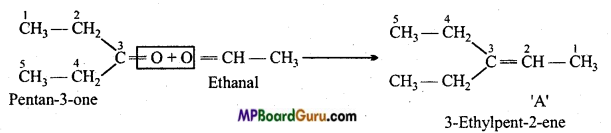
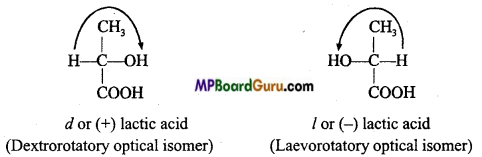
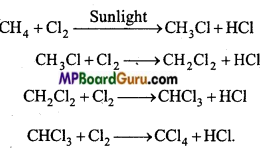
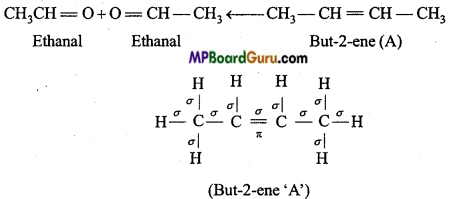
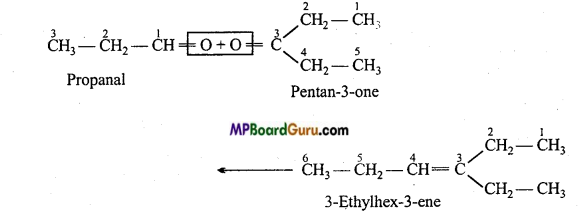

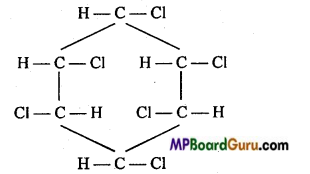
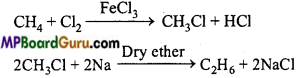
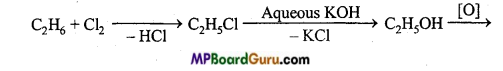
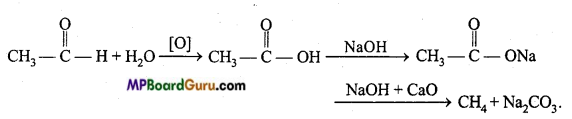
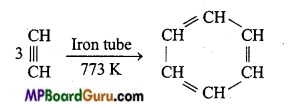
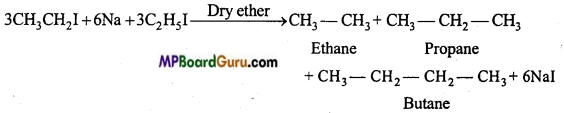

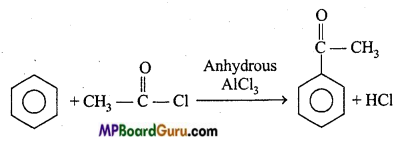

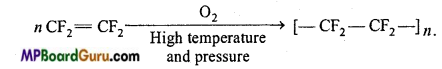
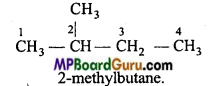
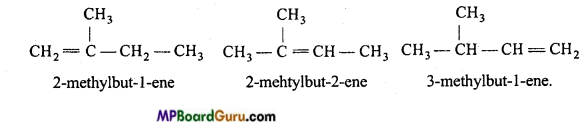
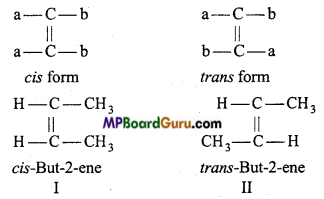
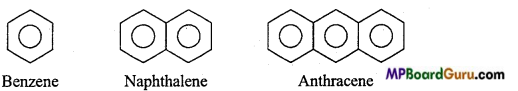

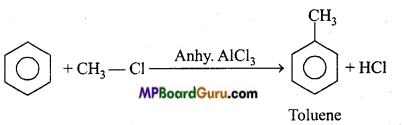
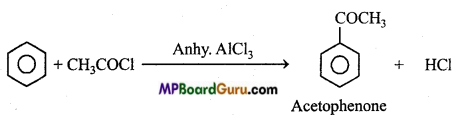

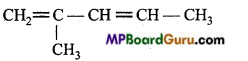
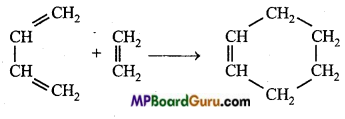

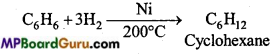
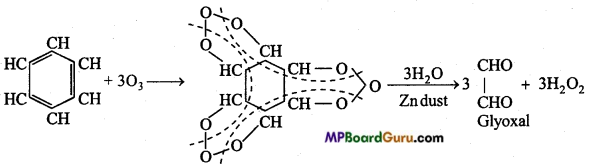
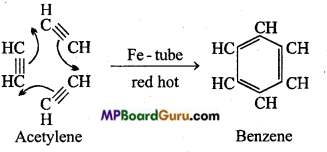
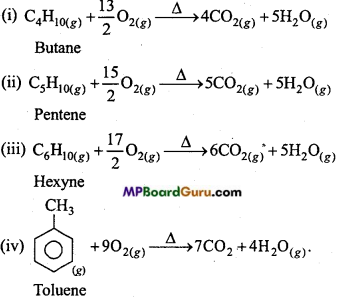

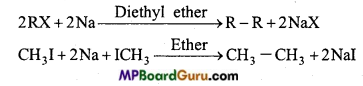
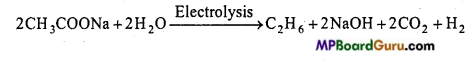
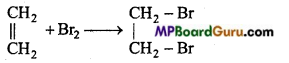
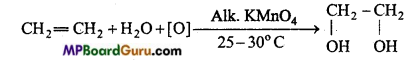
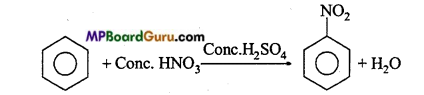

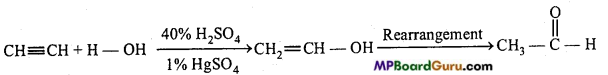
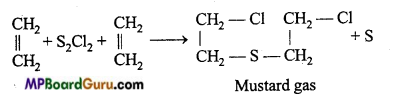
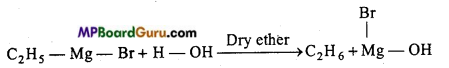
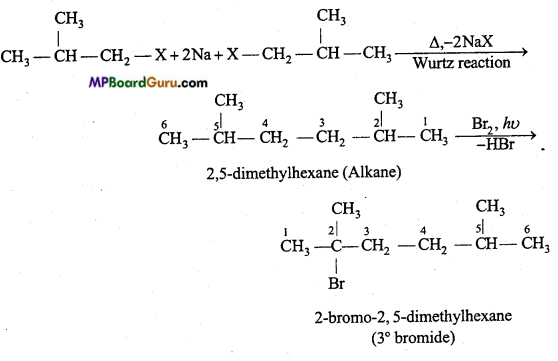
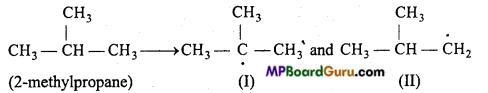
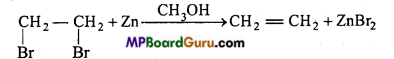
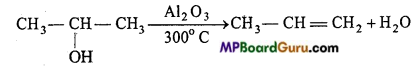
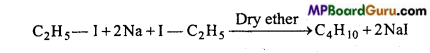

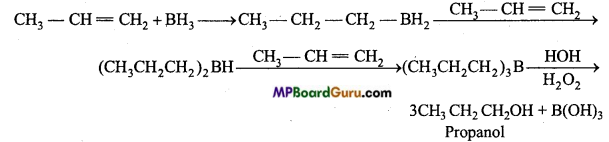
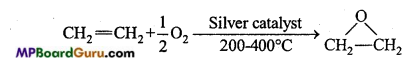
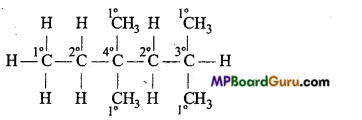


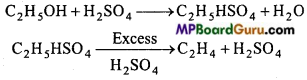

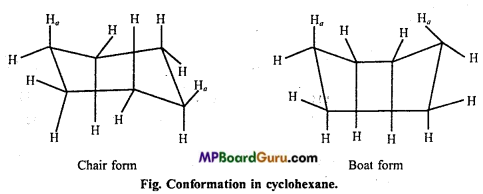
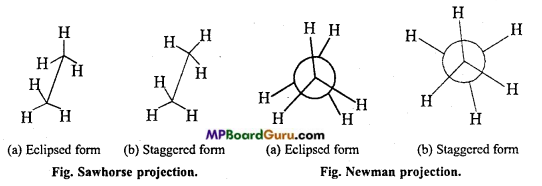
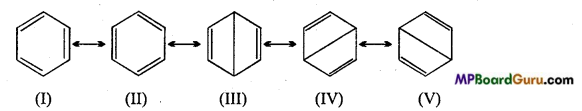
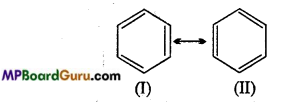
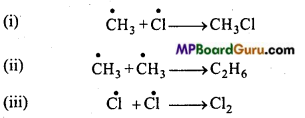
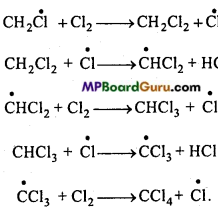
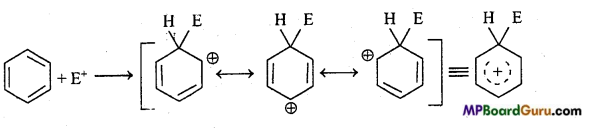
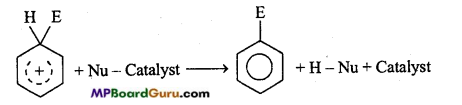

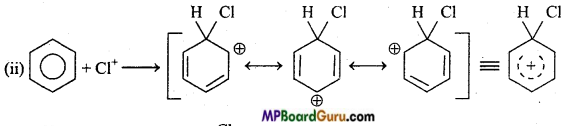
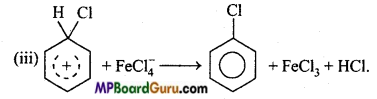
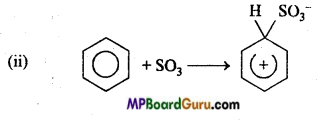
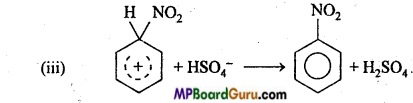
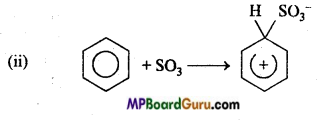
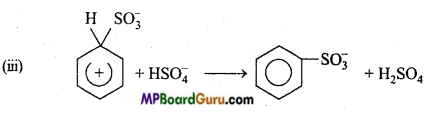
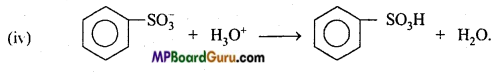

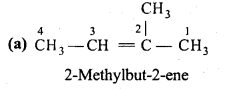
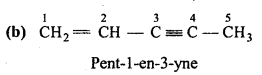
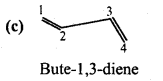
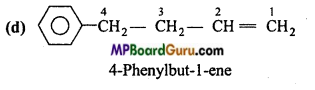
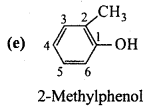
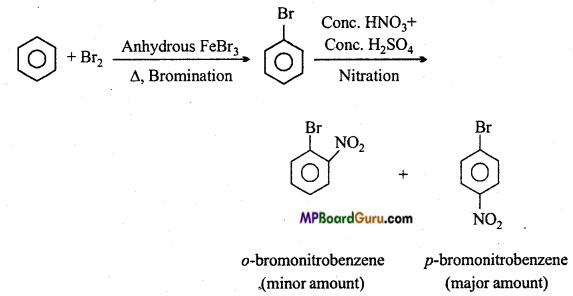
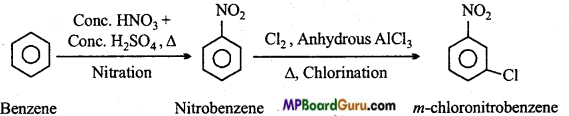
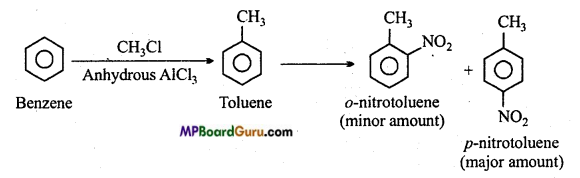
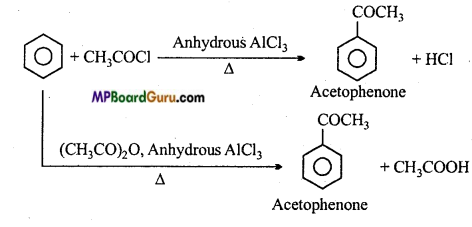
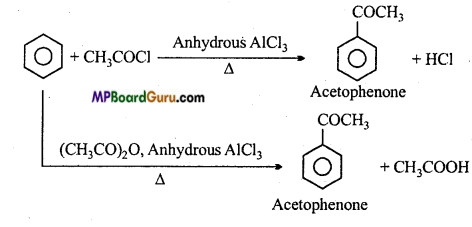
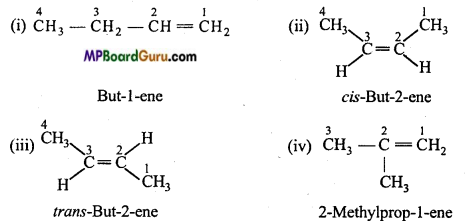

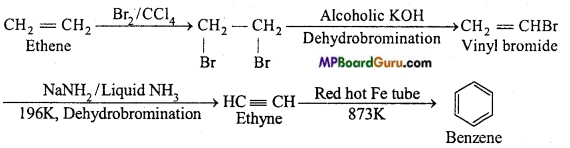
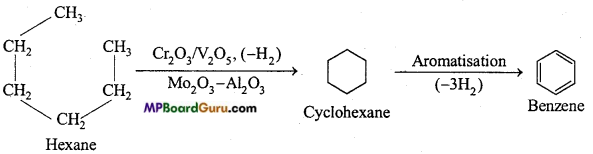


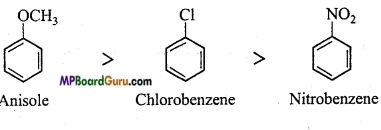
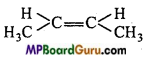
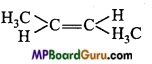
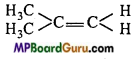
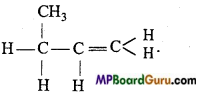
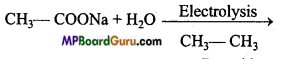
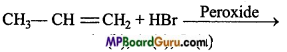
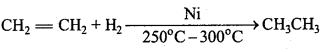 is
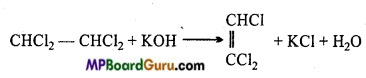
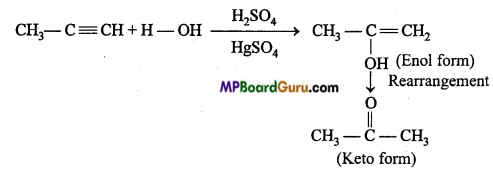
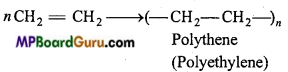
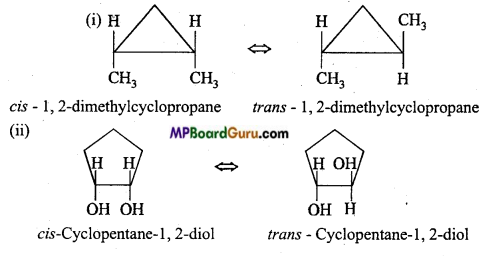
is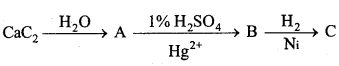 write the name of the products formed in the reaction.
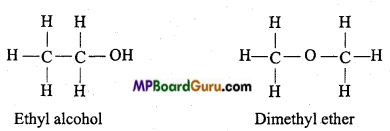
write the name of the products formed in the reaction.