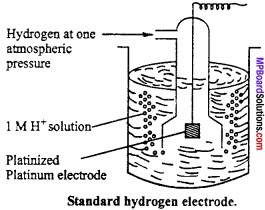
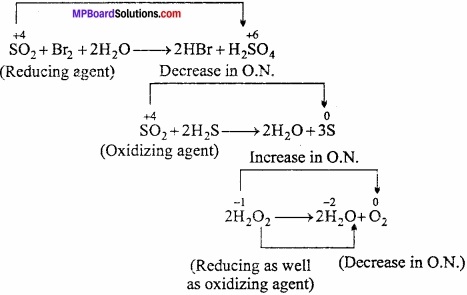
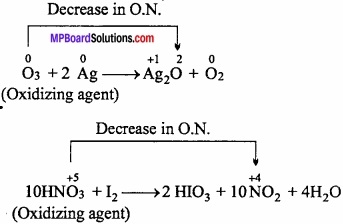
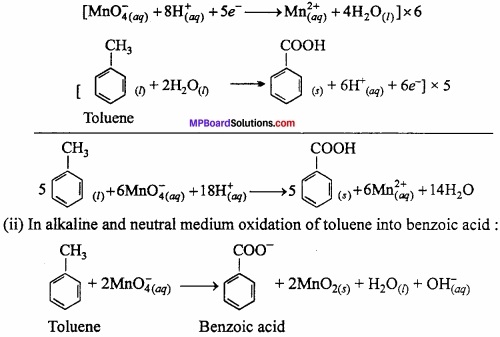
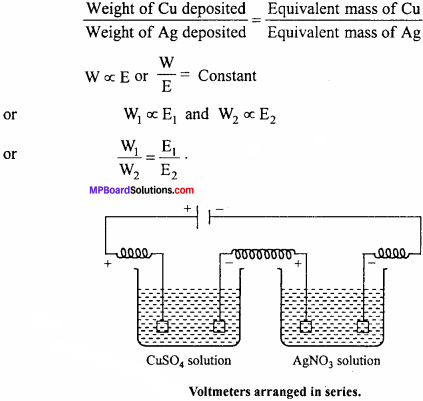
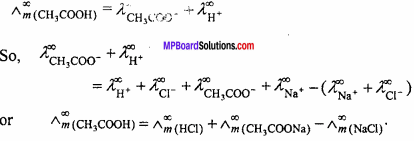
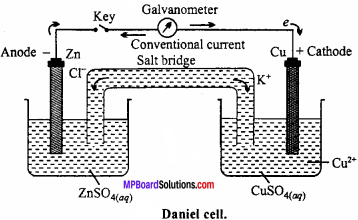
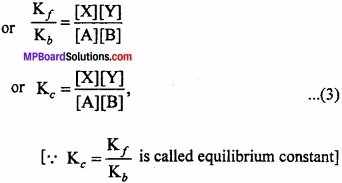
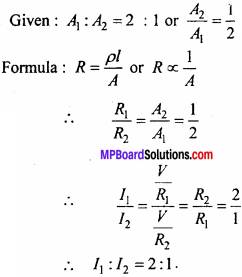
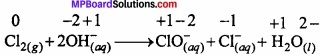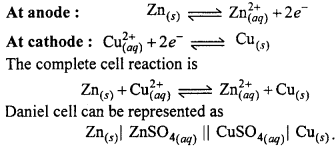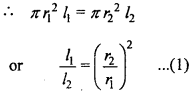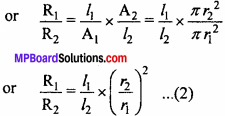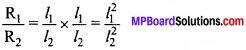MP Board Class 11th Chemistry Important Questions Chapter 9 Hydrogen
Hydrogen Important Questions
Hydrogen Very Short Answer Type Questions
Question 1.
In which compound the oxidation state of hydrogen is negative?
Answer:
CaH2.
Question 2.
The bleaching property of H2O2 is due to oxidation or reduction?
Answer:
H2O2 easily gives oxygen therefore, it is used as bleaching agent. This is due to oxidation property it is used in bleaching.
H2O2 → H2O + [O].
Question 3.
Who discovered hydrogen?
Answer:
Henry Cavendish.
![]()
Question 4.
What is used as moderator in atomic reactors?
Answer:
Heavy water (D2O).
Question 5.
H2O2 reduces Cl2 in which compound?
Answer:
In HCl.
Question 6.
The bleaching property of H2O2 depends upon?
Answer:
Oxidation.
Question 7.
Which oxide forms H2O2 with dii. HCI?
Answer:
Na2O2 and BaC2.
![]()
Question 8.
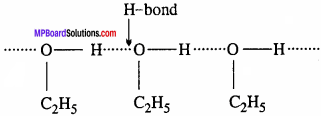
Why the vapourisation of ethanol takes place faster than water?
Answer:
Due to weak hydrogen bonding.
Question 9.
Which type of compounds formed lattice carbides?
Answer:
d and f – block elements.
Question 10.
What is the use of lattice hydrides?
Answer:
For storage of H2 and to catalyze the hydrogenation reaction.
Question 11.
Which acts as propellant in rockets?
Answer:
H2O2 (Hydrogen peroxide).
Question 12.
What is known as the absorbtion of hydrogen by palladium?
Answer:
Absorption.
![]()
Question 13.
Which chemical compound is called calgon?
Answer:
Sodium hexa metaphosphate.
Question 14.
What is main difference between ortho and meta hydrogen?
Answer:
Nuclear Spin.
Question 15.
What is prepared by the combustion of kerosene?
Answer:
Oil gas.
Question 16.
What is used as trace to study the processes occuring in organisms?
Answer:
Heavy water.
Question 17.
What is the radioactive isotope of hydrogen?
Answer:
Tritium.
Question 18.
What is formed by the reaction of calcium phosphate with water?
Answer:
Phosphene.
![]()
Question 19.
What is the bond angle in H – O – O in H2O2?
Answer:
97°.
Question 20.
Which hydrides are not in simple proportion?
Answer:
Interfacial hydrides.
Hydrogen Short Answer Type Questions – I
Question 1.
What is the reason for the temporary and permanent hardness of water? Explain?
Answer:
The temporary hardness of water is due to calcium and magnesium carbonate and the permanent hardness is due to presence of calcium chloride, magnesium chloride, calcium sulphate and magnesium sulphate.
Question 2.
Write an expression to show the amphoteric nature of water?
Answer:
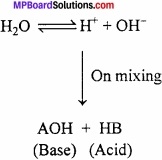
Water has amphoteric nature. It behaves both as acid and base. With strong acid it behaves as base and with strong bases it behaves as acid.
Question 3.
How the impure hydrogen is purified?
Answer:
When hydrogen gas is passed on platinum black or palladium metal, the hydrogen gas is adsorbed this is called adsorption. The impure hydrogen gets purified.
Question 4.
Write the disadvantages of hard water?
Answer:
- Hard water cannot be used in laboratory and for injection in medical.
- Much quantity of soap is wasted if clothes are cleaned with hard water due to the formation of insoluble calcium and magnesium soap.
- Hard water is unuseful in dyeing and printing.
- Cooking with hard water take longer time and spoil the taste and quality of food.
![]()
Question 5.
Explain the Lane’s method to prepare hydrogen?
Answer:
By passing alternate currents of steam and water gas over red hot iron. The oxidation and reduction processes are alternatively carried out.
Oxidizing stage:
Superheated steam is passed over red hot iron at about 1023 K and 1073K, hydrogen gas is produced and magnetic oxides of iron (Fe3O4) is left.
3Fe + 4H2O → Fe3O4 + 4H2↑.
Question 6.
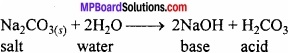
Write the method of removal of permanent as well as temporary hardness of water?
Answer:
By adding washing soda (Na2C03) when permanent hard water is treated with calculated quantity of sodium carbonate solution calcium and magnesium salts present in water get precipitated as insoluble carbonate. Soft water is then decanted off.
CaCl2 + Na2CO3 → CaCO3 + 2NaCl
MgSO4 + Na2CO3 → MgCO3 + Na2SO4
MgCl2 + Na2CO3 → MgCO3 + 2NaCl
CaS04 + Na2CO3 → Na2SO4 + CaCO3.
Question 7.
How the strength of H2O2 is expressed?
Answer:
Strength of Hydrogen Peroxide Solution:
The strength of hydrogen peroxide solution is expressed in terms of the volume of oxygen obtained from it. For example, 10 volume, 20 volume etc. (MPBoardSolutions.com) The strength is equal to the volume of oxygen produced at NTP which is obtained by heating one unit of that solution. The ‘10 volume’ solution of hydrogen peroxide will give 10 ml oxygen at NTP when 1 ml of the sample is heated.
Question 8.
Write the uses of hydrogen?
Answer:
Uses of hydrogen:
- As a reducing agent.
- On account of its lighter nature, it was previously used in filling of balloons and aeroplanes but due to its combustible nature, a mixture of inert gas helium (85%) and hydrogen (15%) is used now.
- In the manufacture of methyl alcohol, ammonia, synthetic petrol and fertilizers.
- For preparing vegetable ghee: Hydrogenation of vegetable oil gives vegetable ghee.
- Oxyhydrogen flame: Hygrogen produces high temperature when bums with oxygen which is used for welding and cutting purposes.
- Hydrogen is used as a rocket propellent.
![]()
Question 9.
What is the difference between the terms “hydrolysis” and “hydration”?
Answer:
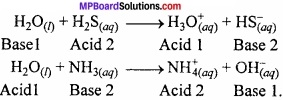
Hydrolysis:
Hydrolysis is the interaction of H+ and OH– ions of water with the anion and cation of salt respectively to form acid and base.
Example:
Hydration:
Hydration is the interaction of water with the salts to form co – ordinated or hydrated ions or hydrated salts.
Example:
1. Water molecules are co-ordinated to metal ion in a complex.
[Cr(H2O)6]Cl3.
2. Water occupying interstitial sites in the crystal lattice.
BaCl2.2H2O.
Question 10.
What is oil gas? How it is prepared?
Answer:
Coal gas is a mixture of many gases. When a thin layer of kerosene is dropped on red hot retort, then big molecules break and converts into methane, ethylene, acetylene. It is used in burners.
Question 11.
What is coal gas? Write its constituent?
Answer:
Coal gas is mixture of many gases where hydrogen gas is main element.
H2 = 43.55%, N2 = 2.12%, CH4 = 25.35%, CO2 = 0.3%, CO = 4.11%, O2 = 0 – 1.5%.
Question 12.
When sodium reacts with water, which gas is released? Write name and formula?
Answer:
The more reactive metals (Na, K, Ca) react with water and metal hydroxide are formed and H2 gas is released.
2Na + 2H – OH → 2NaOH + H2↑
Ca + 2H – OH → Ca(OH)2 + H2↑
2K + 2H – OH → 2K—OH + H2↑.
Question 13.
Write the reason for hardness of water and how many types of hardness are there?
Answer:
Water which does not readily produce lather with soap solution and cause of hardness of water. It is due to the presence of bicarbonate, sulphates and chlorides of calcium and magnesium in it. These salts get dissolved in it as it passes through the rock or ground.
Types of hardness:
- Temporary hardness: It is due to the presence of bicarbonates of calcium, magnesium ion dissolved in water.
- Permanent hardness: It is due to the presence of chlorides and sulphates of calcium, magnesium and iron in water.
Question 14.
What are hard and soft water?
Answer:
Hard water:
Water which does not readily produce lather with soap solution is known as hard water.
Soft water:
Water which readily produce lather with soap solution is known as soft water.
![]()
Question 15.
What is distilled water? Give its one use?
Answer:
The water which is formed with the help of distillation, this water is called distilled water. Its main use is in medicines and to prepare reagents in laboratory.
Question 16.
Write the uses of heavy water?
Answer:
Deuterium oxide is known as heavy water, its chemical formula is D20. It contains two deuterium atom in place of normal hydrogen atom.
Uses of heavy water:
1. Heavy water is used in nuclear reactors to slow down the speed of neutrons to carry out many important reactions. It is also used in the preparation of deuterium D2. It is employed as a tracer in the study of reactions occurring in living organisms.
2. D2O is used in atomic reactors where it serves two purposes:
- It acts as a coolant and
- Act as a moderator.
3. The water of some rivers like the Ganga remains clear and pure even when kept for years. It is due to presence of heavy water in it.
Question 17.
Explain the oxidising and reducing nature of H2O2?
Answer:
1. H2O2 liberates oxygen atom easily so it is strong oxidising agent but when it reacts with other oxidising agent it easily extracts oxygen atom from them hence it possesses reducing property too.
Oxidising nature:
- PbS + 4H2O2 → PbSO4 + 4H2O
- NaNO2 + H2O2 → NaNO3 + H2O
- Na2SO3 + H2O2 → Na2SO4 + H2O
Reducing nature:
- H2O2 + O3 → H2O + 2O2
- H2O2 + Na2O2 → Na2O + H2O + O2
- Ag2O + H2O2 → 2Ag + H2O + O2.
Question 18.
Why the solution H2O2 did not concentrated on heating? How it can be concentrated?
Answer:
Hydrogen peroxide obtained by any method is dilute. It cannot be concentrated by boiling because it decomposes at a temperature below its boiling point. Therefore it is concentrated by following step:
1. Evaporation:
Evaporation of dilute solution of H2O2 on water bath at 70°C gives 45 – 50% H2O2 solution.
2. Vacuum evaporation:
The evaporation is further carried on, in a vacuum desiccator over concentrated sulphuric acid. In this way 66% solution of hydrogen peroxide is obtained.
3. Distillation under reduced pressure:
66% solution of hydrogen peroxide on distillation under reduced pressure, yields hydrogen peroxide of 99% concentration.
4. Crystallization:
H2O2 solution obtained in the above step is placed in a freezing mixture of solid CO2 and ether. Crystals of H2O2 formed are separated and melted to obtain pure H2O2.
![]()
Question 19.
For the manufacture of hydrogen peroxide from peroxides, phosphoric acid is more useful than sulphuric acid why?
Answer:
H2SO4 acts as catalyst in the decomposition of H2O2. So for the manufacture of H2O2 from peroxides instead of H2SO4, weak acids like H3PO4, H2CO3 etc. are more useful.
3BaO2 + 2H3PO4 → Ba3(PO4)2 + 3H2O2
insoluble.
Question 20.
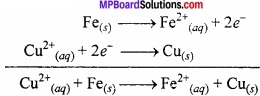
An ionic crystal of an alkali metal has significant covalent character and is almost unreactive towards oxygen and chlorine. This is used in the synthesis of other useful hydrides. Write the formula of this hydride? Write its reaction with Al2Cl6?
Answer:
The hydride is LiH. Due to Li it behaves as covalent compound. It is highly stable.
8LiH + Al2Cl6 → 2LiAlH4 + 6LiCl
Question 21.
Why hard water does not give fast lather with soap?
Answer:
In hard water bicarbonate, chlorides and sulphates of Ca and Mg are present. Soaps are sodium salt of higher fatty acids e,g., sodium stearate (C17H35COONa). (MPBoardSolutions.com) Salts of calcium and magnesium react and form precipitates of calcium and magnesium stearate.
2C17 H35 COONa + M2+ → (CH17 H35 COO)2 M + 2Na+
Until all the salts are precipitated, no lather is formed with soap and this soap is wasted.
Question 22.
Write four uses of H2O2?
Answer:
- It has antiseptic properties and hence used cleaning teeth, ears, wounds, etc.
- It is used for bleaching hair, silk, wool, feathers, ivory, etc.
- As oxidizing agent in laboratory.
- In the preservation of milk, wine and some other drinks.
- As a rocket fuel (as propellant) by producing oxygen.
Question 23.
Write the method of production of hydrogen from water gas?
Answer:
Process of preparation of hydrogen from water gas is known as Bosch process. Bosch process : In this process, hydrogen is obtained by separating it from water gas. Water gas is produced by passing steam over red hot coke.
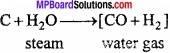
Water gas is mixed with steam and heated at a temperature of 450 °C in presence of Fe2O3 as catalyst and chromic oxide as promoter when carbon monoxide gets oxidized to carbon dioxide.
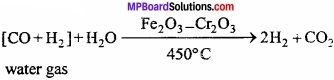
The mixture of hydrogen and carbon dioxide is passed through water under 25 atmospheric pressure. Carbon dioxide gets dissolved leaving behind hydrogen. (MPBoardSolutions.com) Hydrogen so obtained still contains some carbon monoxide. It is removed by passing the gas through ammoniacal solution of cuprous chloride. The hydrogen so obtained contains nitrogen as impurity.
Question 24.
What is conducting water? Write their uses?
Answer:
Kohlrausch distilled the water 42 times at low pressure in an aperture made up of quartz. This water is called conducting water. This is used for
conductivity.
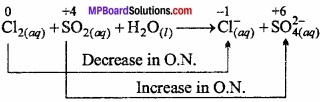
Question 25.
Write the redox reaction between fluorine and water?
Answer:
Fluorine is strong oxidising agent. It oxidizes H2O into O2 and O3.
2F2(g) + 2H2O(l) → O2(g) + 4H+(aq) + 4F–(aq) 3F2(g) + 3H2O(l) → O3(g) + 6H+(aq) + 6F–(aq).
![]()
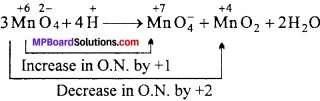
Question 26.
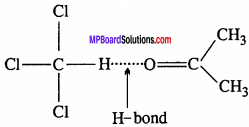
Explain why HCI is a gas and HF is a liquid?
Answer:
F is smaller and more electronegative than Cl, so it forms stronger H – bonds as compared to Cl. As a consequence, more energy is needed to break the H – bonds in HF than HCI and hence the boiling point of HF is higher than that of HCI. That’s why HF is liquid and HCI is a gas.
Question 27.
Write an equation for the manufacturing of D2O2?
Answer:
On reaction of D2SO4 with BaO2, D2O2 is prepared.
BaO2 + D2SO4 → BaSO4 + D2O2
Question 28.
Why H2O2 cannot be kept for a longer time?
Answer:
Since hydrogen peroxide decomposes on storage, therefore H2O2 is stored in brown bottle to avoid the effect of light. This is because the light cause the decomposition of H2O2. (MPBoardSolutions.com) A small quantity of acetanilide is also added which retard the decomposition of H2O2. Here acetanilide acts as an inhibitor.
Question 29.
Why H2O2 is called Antichlor?
Answer:
In neutral medium H202 reduce halogen to acid, metal oxides to metals and ozone to 02.
Cl2 + H2O2 → 2HCl + O2
Br2 + H2O2 → 2HBr + O2.
Due to its ability to reduce chlorine it acts as an Antichlor in bleaching by destroying the unreacted chlorine.
Question 30.
When the temporary hard water is boiled with lime water, it becomes soft Why?
Answer:
This method is called Clark method. When temporary hard water is treated with calculated quantity of lime, bicarbonate present in water change to insoluble carbonates which settle down. Soft water is then decanted off.
Ca(HCO3)2 + Ca(OH)2 → 2CaCO3 + 2H2O
Mg(HCO3)2 + Ca(OH)2 CaCO3 + MgCO3 + 2H2O
![]()
Question 31.
Do you expect the carbon hydrides of the type (Cn H2n+2) to act as Lewis acid or base? Justify your answer?
Answer:
CnH2n+2 such as CH4, C2H6 etc. neither act as Lewis acid nor Lewis base. It is because octet of all the carbon atoms are completed.
Question 32.
Explain the effect of high enthalpy of H – H bond on reactivity of dihydrogen?
Answer:
Due to high enthalpy of dissociation of H – H bond. Hydrogen is unreactive at room temperature. But at high temperature and in presence of catalyst it forms hydrides with metals and non – metals.
Question 33.
In which compound the oxidation number of hydrogen is negative?
Answer:
When hydrogen reacts with higher metal or highly reactive metals like Na, K, Ca etc then electrovalent hydrides are formed. In this hydride the hydrogen has negative oxidation state.
Hydrogen Short Answer Type Questions – II
Question 1.
What is deuterium? Write its two uses?
Answer:
Deuterium is obtained by electrolysis of heavy water. It is collected at cathode and shows reaction similar to hydrogen like its form heavy ammonia (ND3) with nitrogen heavy water (D2O) with oxygen.
![]()
Uses:
- Chemical and Biological reaction.
- It is used in artificial transmutation as target particle.
![]()
Question 2.
Give reason:
- Lakes freeze from top towards bottom.
- Ice floats on water.
Answer:
- Water has maximum density at 4°C. In severe cold, the surface of the lake almost freezes but below the surface, there is water at a temperature about 4°C. This property is extremely helpful for animals living under lake water.
- Ice floats on liquid water as its density is less than liquid water.
Question 3.
If same mass of liquid water and a piece of ice is taken, then why is the density of ice less than that of liquid water?
Answer:
The mass per unit volume (i.emass/volume) is called density. Since water expands on freezing, therefore, volume of ice for the same mass of water is more than liquid water. In other words, density of ice is lower than liquid water and hence ice floats on water.
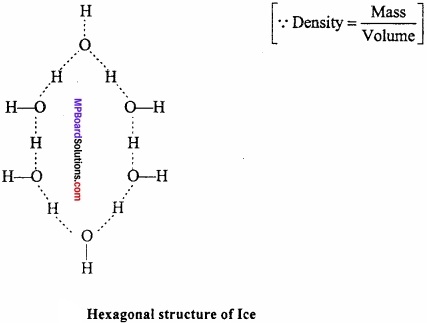
Question 4.
How is the detection of hydrogen peroxide is performed?
Answer:
Test for hydrogen peroxide:
- When few drops of H2O2 is added to acidified potassium dichromate solution containing ether, blue colour solution is formed which confirms the presence of H2O2.
- Titanium dioxide is mixed with hot and cone. H2SO4 and then cooled followed by addition of few drops of hydrogen peroxide, yellow – orange pertitanic acid is produced.
- On adding few drops of H2O2 to acidified glycol solution, blue colour is obtained.
- Addition of few drops of H2O2 to a solution of FeSO4 and starch and potassium iodide solution gives blue colour.
- Addition of few drops of H2O2 to a mixture of aniline and potassium chlorate in dilute sulphuric acid give violet solution.
Question 5.
On the basis of electronic configuration justify the position of hydrogen in periodic table?
Answer:
Hydrogen is the first and the lightest element of the periodic table. It is not a metal but a non – metallic element. But on the basis of electronic configuration it is kept in first group of 5 – block. It is found in atomic form only at high temperature. (MPBoardSolutions.com) In elemental form it is found as diatomic molecule i.e., as H2 and is also called as dihydrogen. One proton and one electron is found in hydrogen atom. Hydrogen forms large number of compounds and is an element of high industrial importance.
Hydrogen is the first element of periodic table with one proton in the nucleus and one electron in the first shell (K – shell). It is not possible to assign a to hydrogen in Mendeleev’s and modem periodic table because it shows similarities and dissimilarities with alkali metals (IA group), halogens (VIIA group) and carbon group (IV A group). This makes position of hydrogen very controversial. Because of this it is also known as Rogue element.
![]()
Question 6.
H2O2 is used for shining the old oil paintings?
Answer:
Old oil paintings contain basic lead oxide, H2S present in atmosphere change lead oxide into lead sulphide. Thus, white paintings become black.

When the paintings are washed with hydrogen peroxide black lead sulphide oxidised into lead sulphate and painting shine again.

Question 7.
Draw the atomic structures of all isotopes of hydrogen?
Answer:
Atoms of an element having same atomic number but different atomic mass is known as isotope. Hydrogen has three isotopes :
- Protium or ordinary hydrogen \(_{ 1 }^{ 1 }\)H
- Deuterium or heavy hydrogen \(_{ 1 }^{ 1 }\)H
- Tritium or radioactive hydrogen \(_{ 1 }^{ 3 }\)H
Structural diagram:
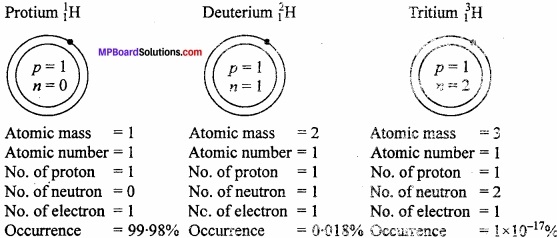
Isotopic effect:
The difference in properties mass is called isotopic effect.
Question 8.
On the basis of structure and chemical reactions? Explain, what are the characteristics of electron deficient hydrides?
Answer:
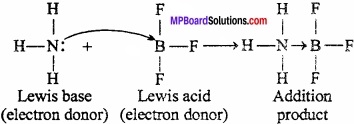
Electron deficient hydrides do not have sufficient number of electrons to form normal covalent bonds. They generally exist in polymeric forms such as B2H6, B4H10, (AlH3)n etc.
Due to deficiency of electrons, these hydrides act as Lewis acids and thus, form complex entities with Lewis bases such as NH3, H– ions.
B2H6 + 2NH3 → BH2(NH3)2]+(BH4)–
B2H6 + 2NaH → 2Na+(BH4)–
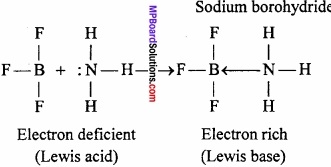
Question 9.
Describe ortho and para hydrogen. How will you obtain para hydrogen?
Answer:
Hydrogen atom consists of one proton in the nucleus and one electron in the extra nuclear part. Both electron and nucleus spin about their own axis. (MPBoardSolutions.com) When two atoms of hydrogen combine to form a hydrogen molecule, the spins of electron should be in opposite direction but the spins of nuclei may either be in the same direction or in opposite directions. When nuclear spins are in same direction, it is called ortho hydrogen. When nuclear spins are in opposite directions, it is called para hydrogen.

Preparation of para hydrogen:
Para hydrogen can be obtained from ordinary hydrogen by keeping it in a quartz vessel with active charcoal at 20K for about 3 to 4 hours.
Question 10.
Why, hard water does not used in boiler?
Answer:
1. Hard water is not used in boiler because compound of calcium and magnesium are deposited on internal side of boiler. This coating is bad conductor of heat so wastes of fuel take place. Due to this coating boiler have to the heated very much for heating water.
2. The boiler may burst if sudden cracks appear in boiler scale. Due to these sudden cracks, the water that comes in contact with the heated boiler is at once converted into steam and the sudden increase in pressure may cause the boiler to burst.
3. Hydrogen is volatile gas therefore, it is risk for explosion.
![]()
Question 11.
How the production of dihydrogen obtained from coal gasification can be increased?
Answer:
The gasification of producing syn gas from coal or coke is called coal gasification.

This is called water gas shift reaction. The CO2 thus produced is removed by scrub-bing with sodium arsenite solution.
Question 12.
Expiain the structure of hydrogen peroxide?
Answer:
Dipole moment of H2O2 is 2.1 D, it indicates that structure of H2O2 is non – planar. By X – rays and other physical methods it is known that structure of H2O2 is like open book. (MPBoardSolutions.com) In gaseous state planes form an angle of 111.5°. In the axis, there are two oxygen atoms and one hydrogen in each plane.
The H – O and O – O bond lengths are 0.95Å and 147Å respectively. There is a slight change in the crystalline state due to hydrogen bonding. The angle between the planes is 90.2°, angle O – O – H = 101.9°, bond length H – O = 0.99Å and O – O is 1.46Å.
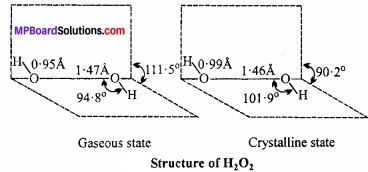
According to modem and latest belief probably both forms occur in equilibrium in aqueous solutions involving ionisation which explains the feeble acidic nature of hydrogen peroxide.
Question 13.
What is water gas? Write its constituents and uses?
Answer:
Bosch processes:
When steam is passed over red hot coke, water gas is produced.

Water gas is mixed with steam and passed over Fe2O3 oxidized to CO2.

This mixture of CO2 and H2 is passed in water at 25 atmospheric pressure CO2 gas is absorbed and H2 gas remains.
Constitution:
H2 = 49%, N2 = 4%, CO = 44%, CO2 = 2.7%, CH4 = 0.3%
Uses:
- As fuel in furnaces
- For preparation of carbonated water gas
- For manufacturing of ammonia.
Question 14.
What do you understand by the term “non – stoichiometric” hydrides? Do you expect this type of the hydrides to be formed by alkali metals? Justify your answer?
Answer:
The hydrides in which the ratio of the metal and hydrogen is found to be fractional are called non – stoichiometric hydrides. It is also observed that this fractional ratio is not fixed but varies with the temperature and pressure. (MPBoardSolutions.com) Such hydrides are formed by d and f – block elements. Usually all the holes are not occupied i.e., some holes always remain vacant and thus, these compounds are non – stoichiometric. Alkali metals do not form non – stoichiometric hydrides as each sodium atom loses its valency electron which is accepted by hydrogen atom to form H– ion. In this way, an ionic compound, Na+ H–, is formed.
![]()
Question 15.
What is Calgon? How hardness of water is removed by using it?
Answer:
Calgon is a trade name for the complex salt (NaPO3)6 as Na2 [Na4 (PO3)6]. When calgon is added to hard water are rendered ineffective due to the formation of water soluble complex ion. This is known as sequstration.
Na2[Na4(PO3)6] + 2CaCl2 → Na2[Ca2(PO3)6] + 4NaCl
Na2 [Na4(PO3)6] + 2MgSO4 → Na2[Mg2 (PO3)6] + 2Na2SO4
The complex calcium and magnesium ion do not form any precipitate with soap. Therefore, they readily form lather with soap solution. Modem detergent contains sodium hexa – meta phosphate to remove the hardness of water due to Ca2+ and Mg2+ ion hence wastage of soap is checked.
Question 16.
Explain that H2O2 acts as both oxidizing and reducing agent. Why?
Answer:
H2O2 liberates oxygen atom easily, so it is strong oxidizing agent, but when it reacts with other oxidizing agent, it easily extracts oxygen atom from them hence, it possesses reducing property too.
Oxidizing nature:
1. 2KI + H2O2 → 2KOH + I2
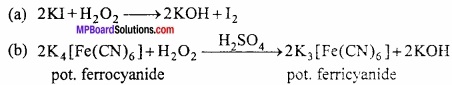
Reducing nature:
(a) In basic medium, it converts ferricyanide to ferrocyanide.
2[Fe(CN6)]3- + 20H– + H2O2 → 2[Fe(CN)6]4- + 2H2O + O2
(b) In acidic medium; it reduces permanganate to manganese (II) salt.
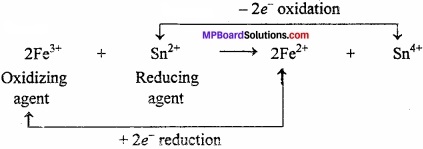
2MnO4– + 6H+ + 5H2O2 → Mn2+ + 8H2O + 5O2
Question 17.
Consider a reaction of water with F2 and suggest, in terms of oxidauon and reduction, which species will oxidized/reduced?
Answer:
2F2(g) + 2H2O(l) → O2(g) + 4H+(aq) + 4F–(aq)
3F2(g) + 3H2O(l) → O2(g) + 6H+(aq) + 6F–(aq)
In these reactions, water acts as a reducing agent as it gets oxidized to either O2 or O3 while fluorine acts as an oxidizing agent as it is reduced to F– ion.
Question 18.
How does H2O2 behave as bleaching agent?
Answer:
Hydrogen peroxide acts as a bleaching agent due to the release of nascent oxygen.
H2O2 → H2O + [0]
The nascent oxygen combines with colouring matter which in turn gets oxidized. The bleaching action of H2O2 is due to the oxidation of colouring matter by nascent oxygen. It is used for bleaching materials like jury, silk, wool, feathers etc.
Question 19.
Why the density of water is maximum at 4°C?
Answer:
Maximum density of water:
Melting of ice decreases hydrogen bonds because cage – like structure is broken. With increase in temperature from 0°C to 4°C with the cleavage of H – bonds, water molecules starts moving closer to each other as a result volume decreases while density increases. (MPBoardSolutions.com) When the temperature becomes greater than 4°C, kinetic energy of H2O molecule increases resulting in expansion of water. As a result of this volume increases while density decreases. That is at 4°C, density of water is maximum.
![]()
Question 20.
What do you understand by
- Electron deficient
- Electron precise and
- Electron rich compounds of hydrogen? Provide justification with suitable examples?
Answer:
1. Electron deficient hydride:
Electron deficient hydrides are those which do not have sufficient number of electrons to form normal covalent bond.
Examples: BH3, AlH3
To makeup their deficiency they generally exist in polymeric form such as B2H6, Al2H6.
2. Electron precise hydride:
Electron precise hydrides are those which have suffi-cient number of electrons required for forming covalent bond.
Examples:
CH4, SiH4 etc.
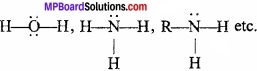
3. Electron rich hydride:
Electron rich hydrides are those which have excesses electron as required to form normal covalent bond. The excesses electron present as lone pair.
Examples: NH3, PH3 etc.
Question 21.
Tell the group of hydrides which include H2O, B2H6 and NaH?
Answer:
- H2O: Covalent or molecular hydride (Electron rich hydride)
- B2H6: Covalent or molecular hydride (Electron deficient hydride)
- NaH: Ionic or salt hydride.
Hydrogen Long Answer Type Questions
Question 1.
Explain Permutit process of removal of hardness of water?
Answer:
Permutit process or Zeolite process:
The water is passed over such substances which readily replace the Ca2+ and Mg2+ ions of hard water by sodium ions. The water does not become hard in presence of sodium salts. Ofcourse too much of sodium salts also make it hard just as in sea water. (MPBoardSolutions.com) The water thus obtained is soft. These products are called as zeolites. Zeolite is a technical name given to certain hydrated silicates of aluminium and sodium. It is also known as permutit. It is obtained by fusing sodium carbonate with alumina and silica.
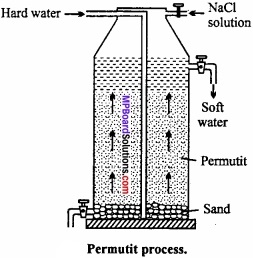
Na2CO3 + Al2O3 + 2SiO2 → Na2Al2Si2O8 + CO2
Na2Al2Si2O8 is known as sodium zeolite and Al2Si2O8 is zeolite and can be represented by Z. Sodium zeolite reacts with chlorides, sulphates of Ca and Mg as given below and hardness is removed.
CaCl2 + Na2 → CaZ + 2NaCl
MgSO4 + Na2 → MgZ + Na2SO2
Reactions have been given by representing sodium zeolite as Na2Z.
After sufficient use permutit gets exhausted and cannot be used further. It is regenerated and made usable by passing 10% solution of sodium chloride.
CaZ + 2NaCl → CaCl2 + Na2Z
MgZ + 2NaCl → MgCl2 + Na2Z
Question 2.
What happens when:
- Calcium hydride reacts with water
- H2O2 is added in acidic KMnO4 solution
- Reaction of potassium ferricyanide with H202
- Reaction of H2 with N2 at high temperature and pressure in presence of Fe?
Answer:
- On reaction of calcium hydride with water, H2 is formed
CaH2 + 2H2O → Ca(OH)2 + 2H2 - The pink colour disappear of KMnO4
2KMnO4 + 3H2SO4 + 5H2O2 → K2SO4 + 2MnSO4 + 8H2O + 5O2 - On adding H2O2 in potassium ferricyanide, it reduces to potassium ferrocyanide
2K3[Fe(CN)6] + 2KOH + H2O2 → 2K4[Fe(CN)6] + 2H2O + O2 - Ammonia is formed when hydrogen reacts with N2 200 atm. pressure

Question 3.
Explain the processes of formation of hydrogen in laboratory?
Answer:
Preparation of hydrogen (laboratory method):
Hydrogen is prepared in laboratory by action of dil. H2SO4 on granulated Zn. Zn is taken into woulf bottle fitted with thistle funnel and a outlet tube.
Hydrogen formed by this method is collected over water by displacement method.
Zn + H2SO4 → ZnSO4 + H2↑
Purification of hydrogen : Hydrogen prepared by this method contains following impurities:
- Arsine (AsH3) and phosphene (PH3)
- H2S
- SO2, CO2 and oxides of nitrogen (NO2)
- Water vapours.
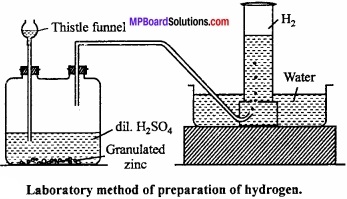
In order to remove these impurities, gas is passed in a series of U – tubes containing different solutions:
- Passed in tube filled with AgNO3 solution to absorb AsH3 and PH3.
- Passed in the tube containing lead nitrate solution to remove H2S.
- Passed in the tube filled with cone. KOH solution to remove SO2, CO2 and NO2.
Question 4.
Rohan heard that instructions were given to the laboratory attendent to store a particular chemical Le., keep it in the dark room, add some urea in it, and keep it away from dust This chemical acts as an oxidizing as well as a reducing agent in both acidic and alkaline media. This chemical is important for use in the pollution control treatment of domestic and industrial effluents?
- Write the name of this compound.
- Explain, why such precautions are taken for storing this chemical?
Answer:
- H2O2 (Hydrogen peroxide).
- The following precautions must be taken while storing hydrogen peroxide:
- It must be kept in wax lined coloured bottles because the rough glass surface, light and dust particles are responsible for its decomposition.
- A small amount of negative catalyst such as urea, glycerol, phosphoric acid etc. is generally added which retards its decomposition.
![]()
Question 5.
Calculate the strength of 5 volume of H2O2 solution?
Answer:
5 volumes 2O2 solution means that 1 volume of this solution will decompose to give 5 volumes of oxygen at S.T.P.

5 L oxygen is obtained from \(\frac{68×5}{22.4}\) g H2O2 = 15.17 g H2O2
i.e. 15g H2O2 is present in 1L solution. Thus, strength of the solution = 15 g/L.
Question 6.
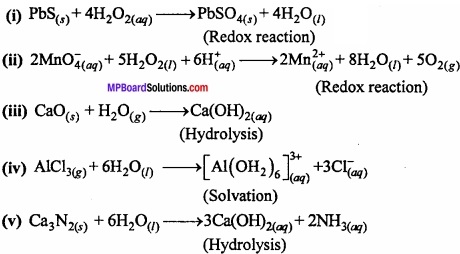
Complete the following reactions:
- PbS(s) + H2O2(aq) →
- MnO4–(aq) + H2O2(aq) →
- CaO(s) + H2O(g) →
- AlCl3(g) + HzO(l) →
- Ca3N2(s) + H2O(l) →
above
- Hydrolysis
- Redox
- Solvation process.
Answer: