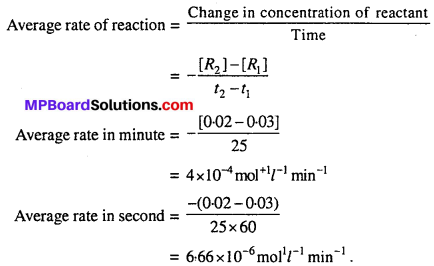
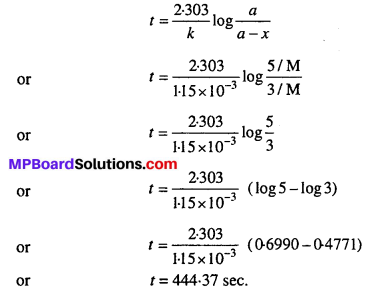
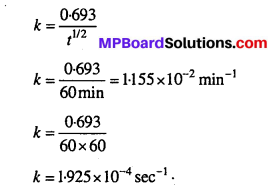
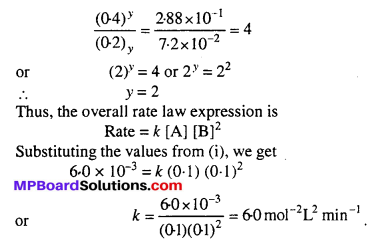
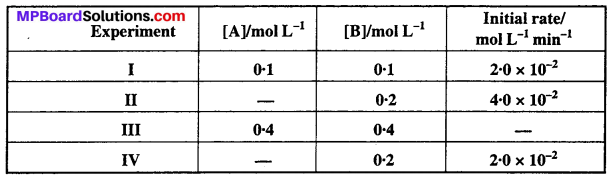
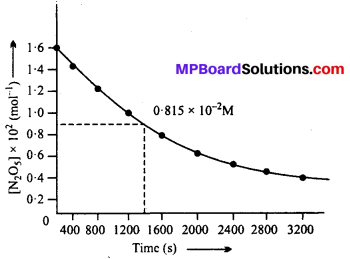
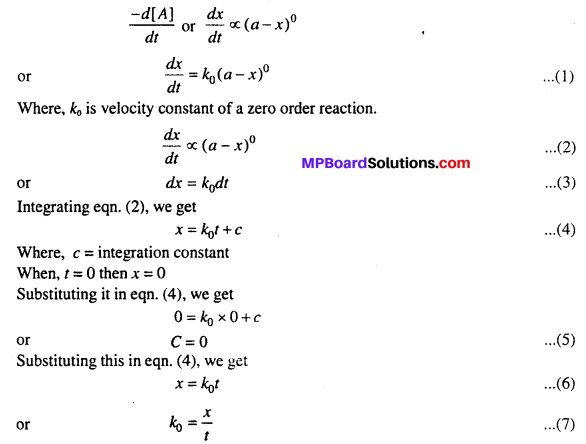
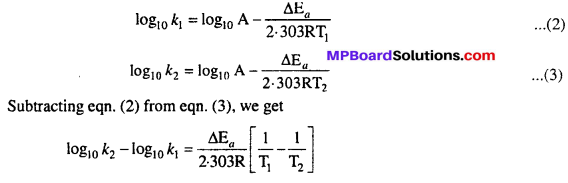
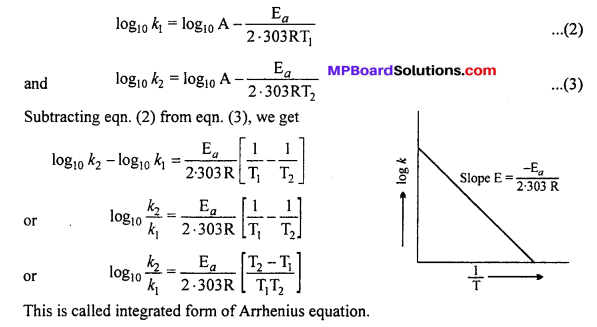
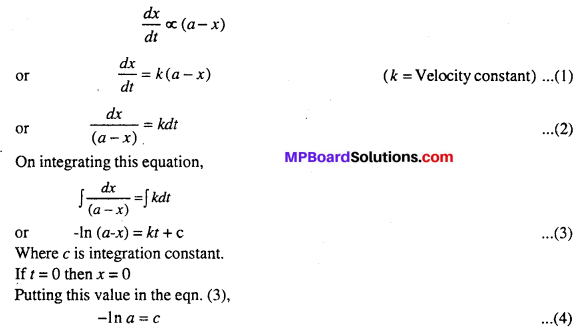
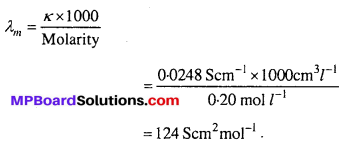
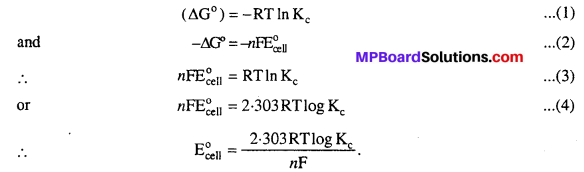
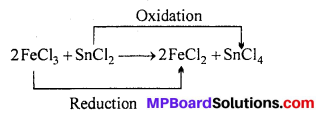
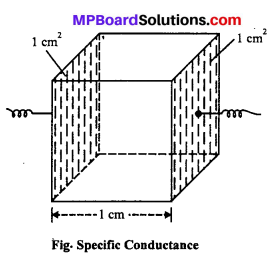
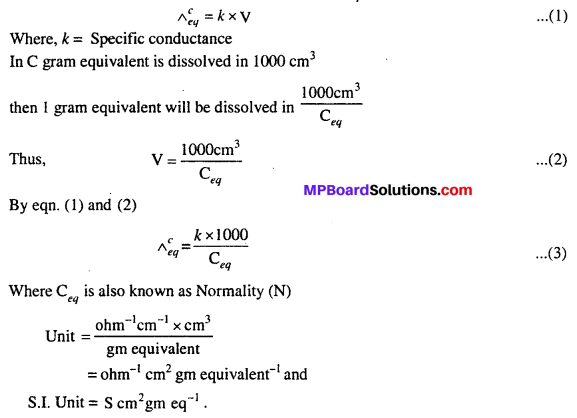
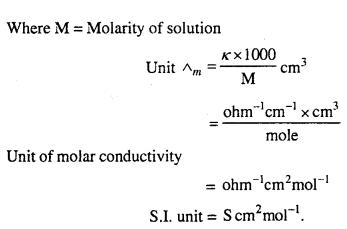
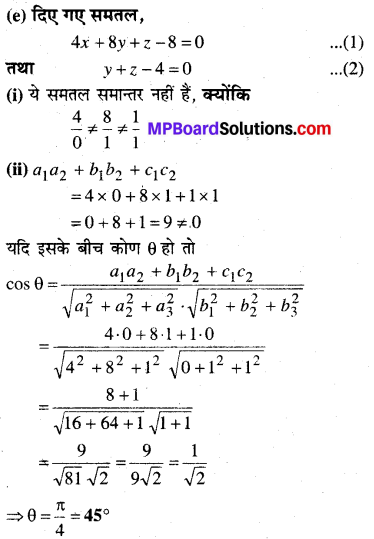
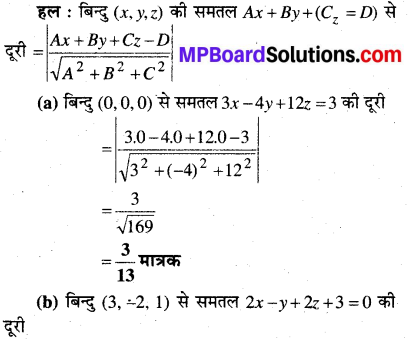
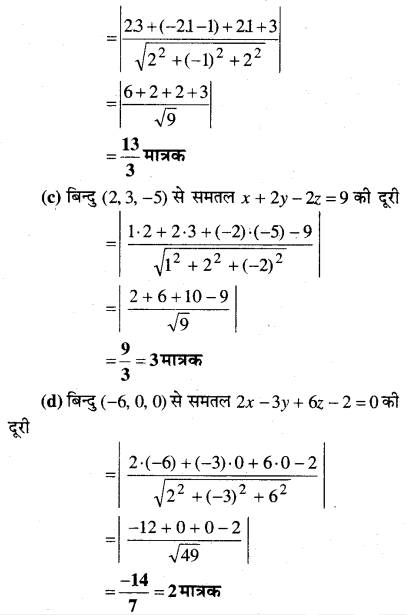
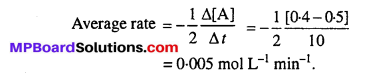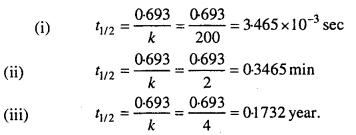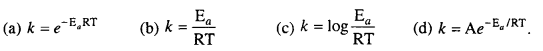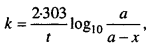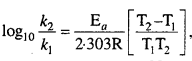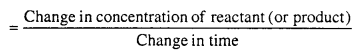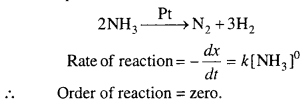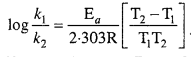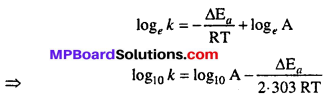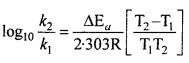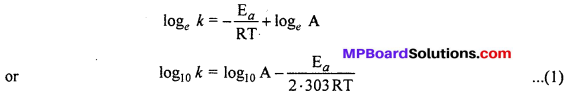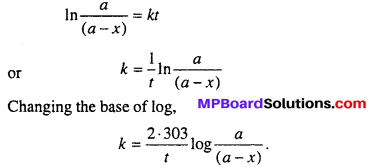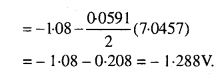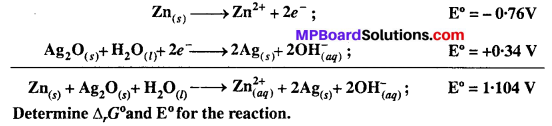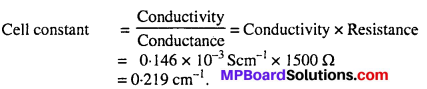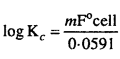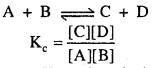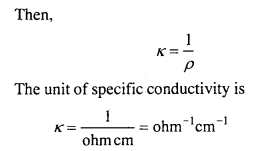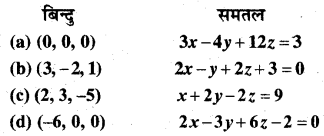MP Board Class 12th Biology Solutions Chapter 1 Reproduction in Organisms
Reproduction in Organisms NCERT Textbook Questions and Answers
Question 1.
Why is reproduction essential (neccessary) for organisms?
Answer:
An organism gives rise to young ones by reproduction. The offspring grow, mature and in turn produce new offspring. Thus, there is a cycle of birth, growth and death. Reproduction enables the continuity of the species, generation after generation. So, therefore reproduction is essential.
Question 2.
Which is a better mode of reproduction sexual or asexual? Why?
Answer:
Sexual mode of reproduction is better because it is biparental reproduction and introduces variation among offsprings and their parants due to crossing over and recombination during gamete formation by meiosis.
Question 3.
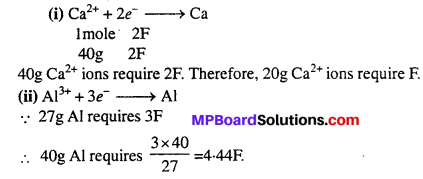
Why is the offspring formed by asexual reproduction referred to as clone?
Answer:
In sexual reproduction, the offspring is morphologically and genetically identical to the parent and to each other. Hence, it is called clone.
![]()
Question 4.
Offsprings formed due to sexual reproduction have better chances of survival. Why? Is this statement always true?
Answer:
Offspring formed due to sexual reproduction have better chance of survival because:
- The offspring consists its hybrid characters which may adapt better with the different environment.
- Genetic variations are introduced among the offspring’s, which increases the biological tolerance.
- Sexual reproduction occurs in adverse condition in lower plant kingdom, so sexual spores survive in adverse condition.
- Sexual reproduction may not always show better chances of survival because the offspring may be inferior to the parents.
Question 5.
How does the progeny formed from asexual reproduction differ from those formed by sexual reproduction?
Answer:
The progenies have similar genetic make up and are exact copies of their parents in asexual reproduction but the progenies have different genetic make up and different from each other and dissimilar to the parent in sexual reproduction.
Variation is absent in asexual reproduction but it is a common phenomenon of sexual reproduction. In asexual reproduction, variation may occur due to mutation whereas varia¬tion occurs due to mutation, crossing over and recombination in sexual reproduction.
Question 6.
Distinguish between asexual and sexual reproduction. Why is vegetative reproduction aslo considered as a type of asexual reproduction?
Answer:
Differences between Asexual and Sexual reproduction:
| S.No. | Asexual reproduction | Sexual reproduction |
| 1. | In this type of reproduction only one parent is required. | In this type of reproduction two parents of different sexes are required. |
| 2. | Whole body or a single cell acts as reproductive unit. | Reproductive units are called as gametes which are produced by specific tissues. |
| 3. | Offsprings remain pure, i.e., alike their parents. | Offsprings differ from their parents. |
| 4. | It occurs by mitosis cell division. | Gametes are formed by meiosis cell division and zygote develops by mitosis cell division |
| 5. | Variation does not occur. | Variation occurs. |
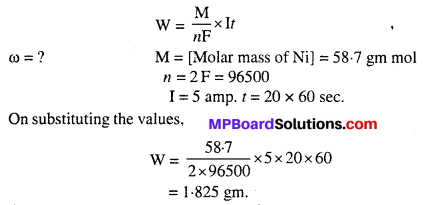
Vegetative reproduction is considered as a type of asexual reproduction because :
- It is uniparental reproduction.
- There is no involvement of gametes or sex cells.
- Cell division and no reductional division takes place.
- Vegetative propagules are somatic cells.
![]()
Question 7.
What is vegetative propagation ? Give two suitable examples.
Answer:
In plants, the vegetative propagules (runner, rhizome, sucker, etc.) are capable of producing new offsprings by the process called vegetative propagation. As the formation of these vegetative propagules does not involve both the parents, the process involved is asexual.
Examples:
- Adventitious buds in the notches along the leaf margins of bryophyllum grow to form new plants.
- Potato tuber having buds when grown, develops into a new plant.
Question 8.
Define:
(a) Juvenile phase
(b) Reproductive phase
(c) Senescent phase.
Answer:
(a) Juvenile phase : It is the pre-reproductive in which all organisms require a certain growth and maturity in the life before reproducing sexually.
(b) Reproductive phase: Reproductive phase is the phase in the life cycle, where an organism possess all the capacity and potential to reproduce sexually. It is the end of juvenile phase or vegetative phase.
(c) Senescent phase : It is the post reproductive phase in the life cycle where an organism slowly losses the rate of metabolism, reproductive potential and show deterioration of the physiological activity of the body.
Question 9.
Higher organisms have resorted to sexual reproduction in spite of its complexity. Why?
Answer:
Higher organisms have resorted to sexual reproduction to :
- Get over the unfavourable condition
- Restore high gene pool in a population
- Restore vigour and vitality of the race and .
- Get proper parental care ‘
- Introduce variation to enable better adaptive capacity.
Question 10.
Explain, why meiosis and gametogenesis are always interlinked.
Answer:
Gametogenesis (formation of male and female gametes) is associated with reduction in chromosome number thus, the gamete formed contains half chromosome set of the parental cell. So, gametogenesis is interlinked with meiosis because in meiosis reduction of chromosome number from diploid set (2n) to haploid set (n) takes place.
Question 11.
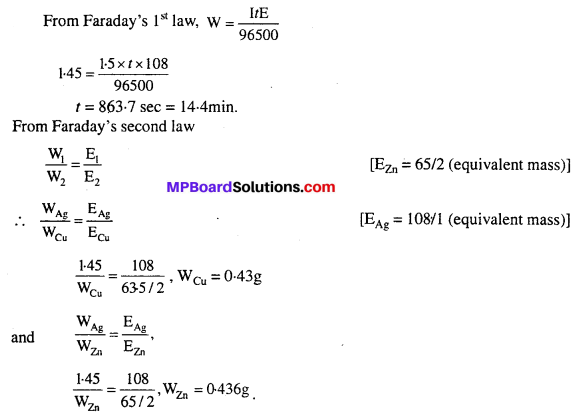
Identify each part in a flowering plant and write whether it is haploid (n) or diploid (2n).
Answer:

![]()
Question 12.
Define external fertilization mention its disadvantages.
Answer:
The fusion of compatible gametes (Male and female) outside the body of an organism is called external fertilization, e.g., in Frog.
Disadvantages of external fertilization:
- It requires a medium for fusion of gametes.
- The young ones are often exposed to the predators.
- After fertilization, offsprings are produce large in number but no parental care is provided
Question 13.
Differentiate between a Zoospore and Zygote.
Answer:
Differences between Monera and Protista:
| S.No. | Zoospore | Zygote |
| 1. | These are endogenously, asexually | Zygote is a diploid cell formed by fusion |
| 3. | Zoospores takes part in dispersal. | Zygote does not have significant role in dispersal. |
| 3. | Zoospores takes part in dispersal. | Zygote does not have significant role in dispersal. |
Question 14.
Differentiate between Gametogenesis and Embryogenesis.
Answer:
Differences between Gametogenesis and Embryogenesis:
| S.No. | Gametogenesis | Embryogenesis |
| 1. | It is the formation of gametes from meiocytes. | It is the formation of embryo from zygote cell. |
| 2 | This is a pre-fertilization event. | This is a post fertilization event. |
| 3. | It occurs inside reproductive organs. | It occurs outside or inside the female body. |
| 4. | It produces haploid gamete. | It gives rise to diploid embryo. |
| 5. | The cell division during gametogenesis is meiotic in diploid organism. | The cell division during embryogenesis is mitotic in diploid organisms. |
Question 15.
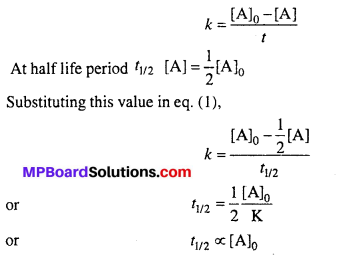
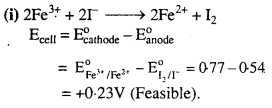
Describe the post-fertilization changes in a flower.
Answer:
The post-fertilization changes that take place in a flower are as follows :
- The formation of zygote which later develops into an embryo and primary endosperm cell which develops into endosperm takes place.
- While the sepals, petals and stamens are shed, the pistil remains intact.
- The fertilized ovule develops into seeds.
- The ovary matures into a fruit that later develops a thick protective wall, called pericarp.
- Seeds after dispersal germinate under favourable conditions which later develop into a new plant.
![]()
Question 16.
What is a bisexual flower ? Collect five bisexual flowers from your neigh bourhood and with the help of your teacher find our their common and scientific names.
Answer:
| S.No. | Common Name | Scientific Name |
| 1. | China rose | Hibiscus rosa sinensis |
| 2 | Chandni | Ervatamia divaricata |
| 3. | Makoy | Solatium nigrum |
| 4. | Sunflower | Helianthus annuus |
| 5. | Mustard | Brassica compestris. |
Question 17.
Examine a few flowers of any cucurbit plant and try to identify the staminate and pistillate flowers. Do you know any other plant that bears unisexual flowers ?
Answer:
Cucurbit plant bears unisexual flowers as these flowers have either the stamen or the pistil. The staminate flowers bear bright, yellow colored petals along with stamens that represent the male reproductive structure. On the other hand, the pistillate flowers bear only the pistil that represents the female reproductive structure. Other examples of plants that bear unisexual flowers are corn, papaya, cucumber, etc.
Question 18.
Why are offspring of oviparous animals at a greater risk as compared to offspring of viviparous animals?
Answer:
In viviparous animals, the young one develops inside the body of the female organism. As a result of this, the young one gets better production and nourishment for proper development. In case of oviparous animals, they lay eggs and the young ones develop inside the calcareous shell, outside the body of the female. So, the young ones are not effectively protected and nourished and are vulnerable, to predators so, they are at a greater risk as compared to the offsprings of the viviparous animals.
Reproduction in Organisms Other Important Questions and Answers
Reproduction in Organisms Objective Type Questions
1. Choose the Correct Answers:
Question 1.
Callose wall is found: (CBSE PMT 2007)
(a) In male gamete
(b) In ovum
(c) In pollen grain
Answer:
(d) In megaspore mother cell.
Question 2.
Name the plant in which new plant arise from the notches of leaves through vegetative propagation: (AFMC2012)
(a) Asparagus
(b) Chrysanthemum
(c) Agave
(d) Bryophyllum.
Answer:
(d) Bryophyllum.
![]()
Question 3.
Which type of vegetative propagation is occurs in banana: (AMU 2012)
(a) From tuber
(b) From rhizome
(c) From bulb
(d) From stolen.
Answer:
(b) From rhizome
Question 4.
Which of the following is viviparous:
(a) Tortoise
(b) Bony fish
(c) Hummingbird
(d) Whale
Answer:
(d) Whale
Question 5.
Which of the following is characteristics of the ‘clone’:
(a) Similar to ancestors in genetic character
(b) A group of plants developed in vegetative propagation
(c) Plants developed from same parents
(d) All of the above.
Answer:
(d) All of the above.
Question 6.
Organism which give birth to young ones:
(a) Viviparous
(b) Amphibians
(c) Oviparous
(d) Coelomate.
Answer:
(a) Viviparous
Question 7.
Set of chromosome found in zygote:
(a) X
(b) 2X
(c) 3X
(d) 4X.
Answer:
(b) 2X
Question 8.
The plant which can be obtained same as parent plant:
(a) Through seeds
(b) Through fruits
(c) By cutting the stem
(d) By hybridization.
Answer:
(c) By cutting the stem
Question 9.
Vegetative propagation in potato is:
(a) By rhizome
(b) By bulb
(c) By stem
(d) By tuber.
Answer:
(d) By tuber.
Question 10.
Which of the following is oviparous animal:
(a) Bat
(b) Whale
(c) Penguin
(d) Amoeba.
Answer:
(c) Penguin
2. Fill in the Blanks:
- Reproductive organs in plants are developed in ……………….. phase.
- ……………….. nucleus are present in pollen tube of angiosperms.
- The ……………….. are not form in Rose and Banana, therefore, reproduction occurs through ………………..
- Reproductive organs of animals are differentiate in ……………….. stage.
- Eichomia propagate through ………………. in water.
- Rate of multiplication is greater ……………….. reproduction than ……………….. reproduction.
Answer:
- Adolescence
- Three
- Seeds, Vegetative propagation
- Embryo
- Offset
- Sexual, Asexual.
![]()
3. Match the Following

Answer:
- (e)
- (d)
- (f)
- (a)
- (c)
- (b)
Answer in One Word/Sentence:
- What we called uniparental structure formed from asexual reproduction which is genetically and morphologically similar.
- Write the name of an algae reproduce by means of zoospores.
- Animal reproduce asexually.
- What we called spores having flagella.
- In Bryophyllum what kind of bud reproduce vegetatively ?
Answer:
- Clone
- Chlamydomonas
- Spongilla
- Zoo-spores
- Adventitious bud.
Reproduction in Organisms Very Short Answer Type Questions
Question 1.
What is meant by life span ?
Answer:
The period from birth to the natural death of an organism is called life span.
Question 2.
Give definition of fertilization.
Answer:
Fertilization is a process in which male and female gamete fused to form the zygote.
Question 3.
What is clone ?
Answer:
Morphologically and genetically similar individuals who are produced by single parent is called clone.
Question 4.
Give the name of an organism in which asexual reproduction occurs through conidia.
Answer:
Penicillum.
![]()
Question 5.
In which plant vegetative propagation occurs through rhizome ?
Answer:
In Ginger, Termeric.
Question 6.
Give the name of an organism in which transverse binary fission occurs.
Answer:Paramoecium.
Question 7.
What is aging ?
Answer:
When humans are not capable for reproduction is known as aging.
Question 8.
Give two examples of Hermaphrodite plants.
Answer:
Cucurbita and Coconut are hermaphrodite plant.