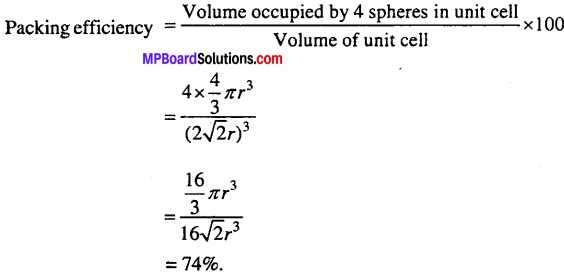
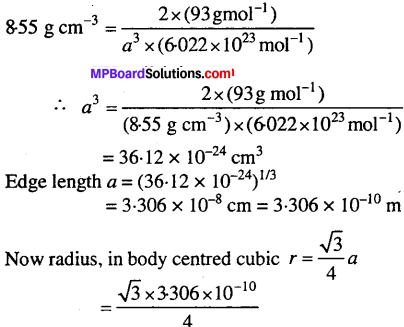
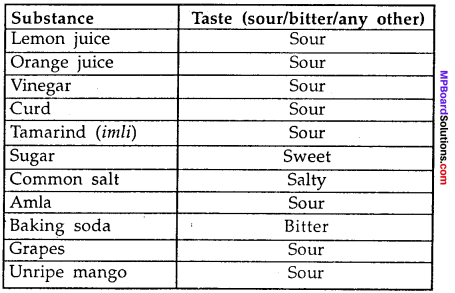
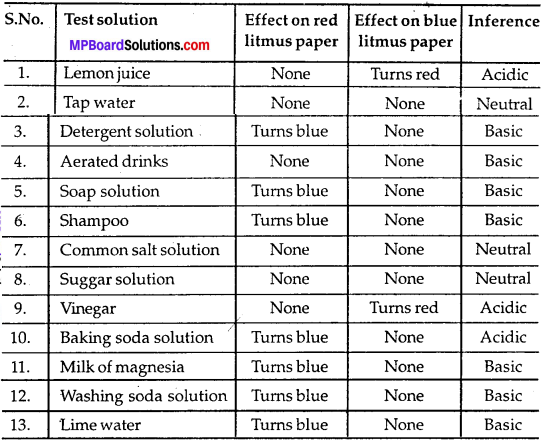
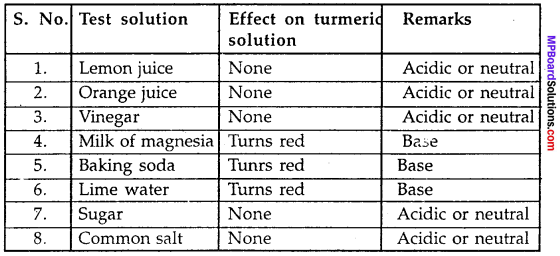
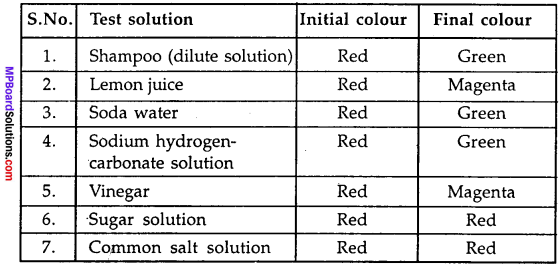
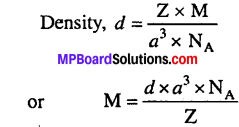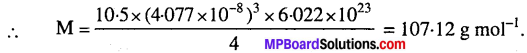MP Board Class 9th Maths Solutions Chapter 11 Constructions Ex 11.2
![]()
Question 1.
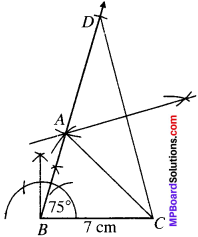
Construct a triangle ABC in which BC = 7 cm, B = ∠75° and AB + AC = 13 cm.
Solution:
BC = 7 cm
∠B = 15°
AB + BC = 13 cm.
Question 2.
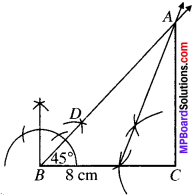
Construct a triangle ABC in which BC = 8 cm, ∠B = 45° AB – AC = 3.5 cm.
Solution:
BC = 8 cm
∠B = 45°
AB – AC = 3.5 cm.
Question 3.
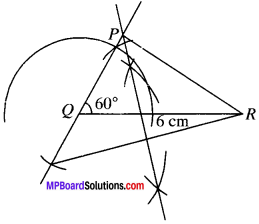
Construct a triangle PQR in which QR = 6 cm. ∠Q = 60° and PR – PQ = 2 cm.
Solution:
QR = 6 cm
∠Q =60°
PR – PQ = 2 cm
Question 4.
Construct a triangle XYZ in which ∠Y = 30°, ∠Z = 90° and XY + YZ + ZX = 11 cm.
Solution:
XY + YZ + ZX = 11 cm
∠Y = 30°
∠Z =90°
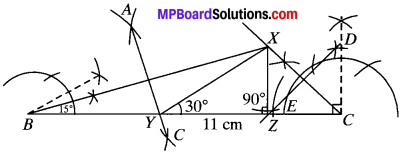
- Draw a line segment BC =11 cm.
- At B construct an angle of 30° and at C, draw angle of 90°.
- Bisect these angles. Let the bisectors of these angles intersect atX.
- Draw perpendicular bisectors AC of BX to intersect BC at Y and DZ of XC to intersect BC at Z.
- Join XY and XZ. XYZ is the required D.
![]()
Question 5.
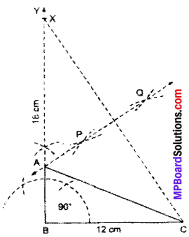
Construct a right triangle whose base is 12 cm and sum of its hypotenuse and other side is 18 cm.
Solution:
Steps of construction:
- Draw \(\overline { BC } \) = 12 cm.
- Construct ∠CBY = 90°.
- From \(\overline { BY } \), cut off BX = 18 cm.
- Join CX.
- Draw PQ, the perpendicular bisector of CX, such that PQ meets BX at A.
- JoinAC.
Thus, ABC is the required triangle.