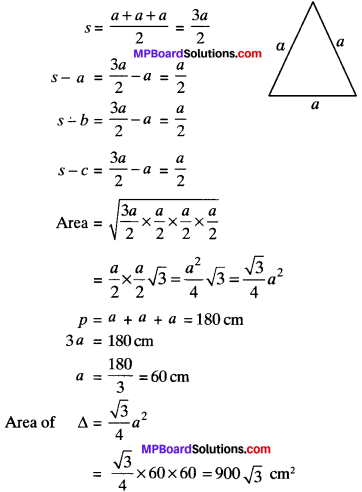
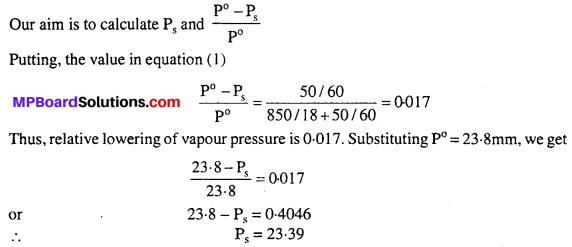
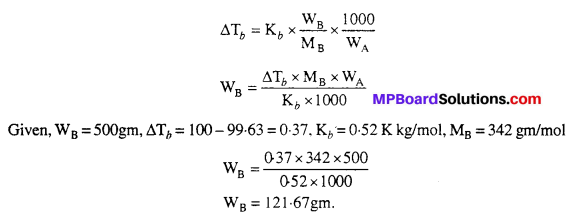
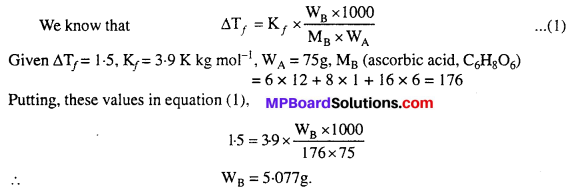
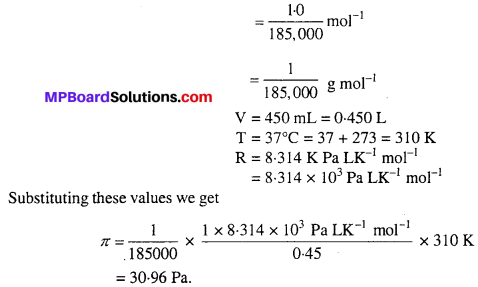
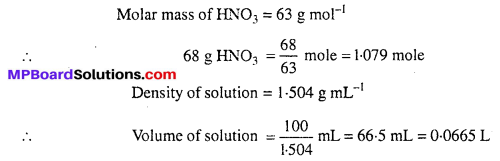
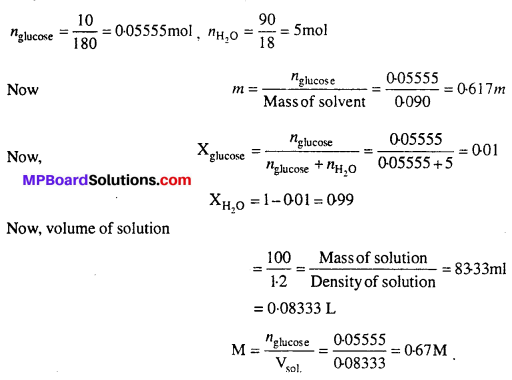
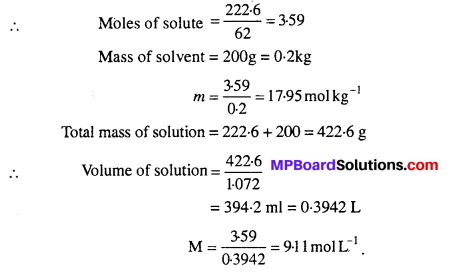
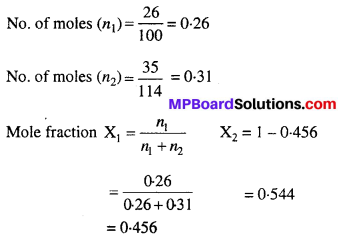
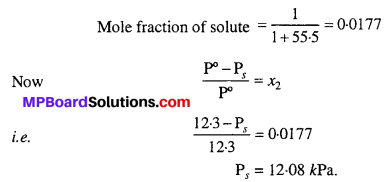
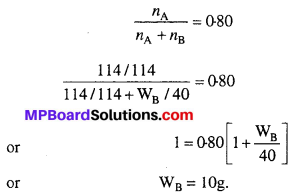
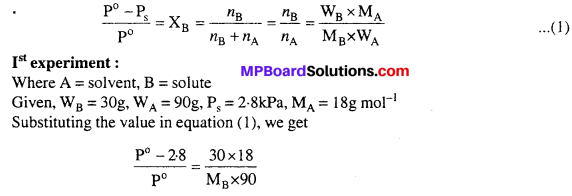
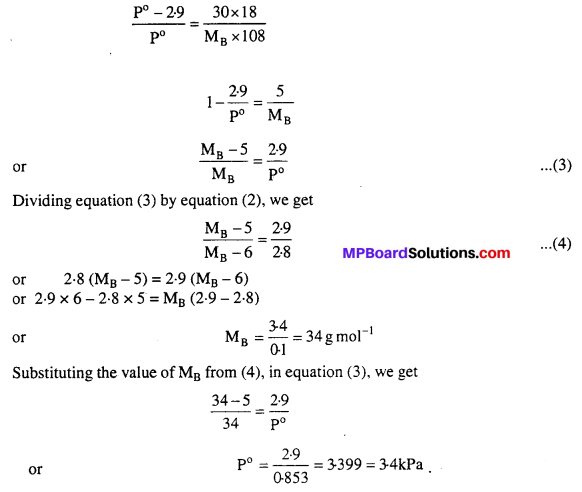
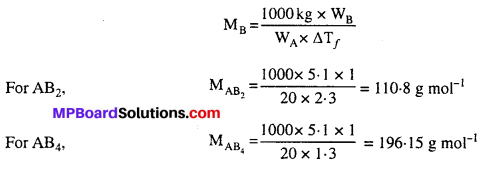
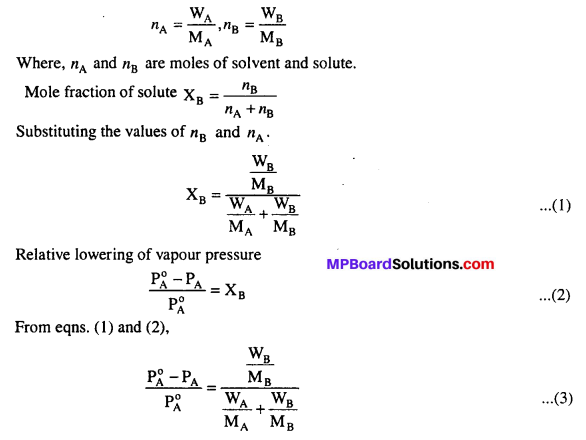
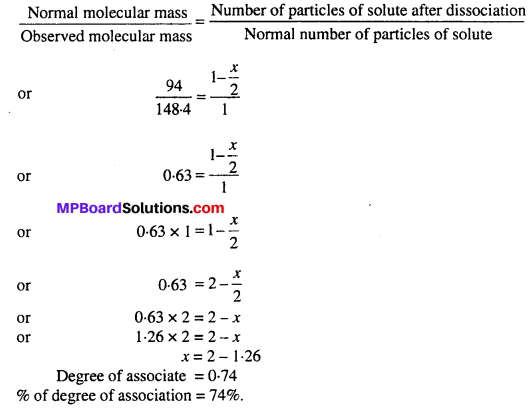
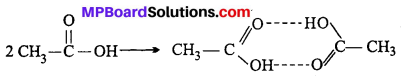
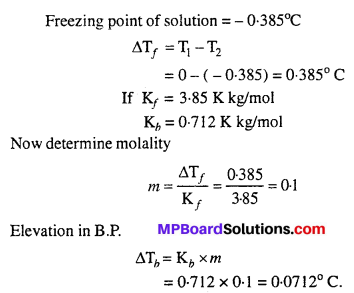
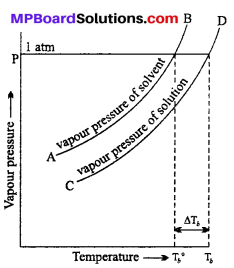
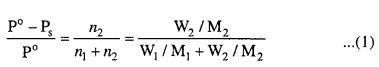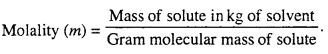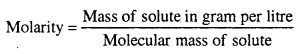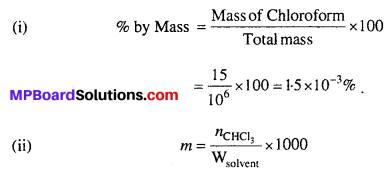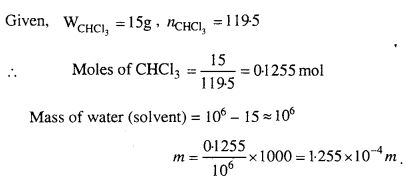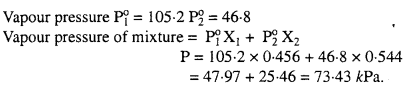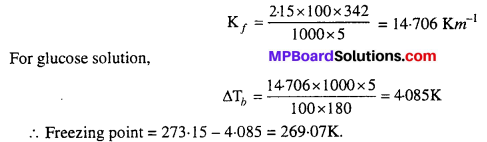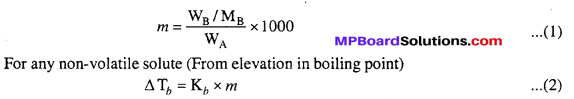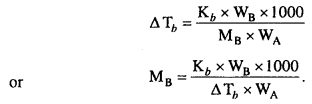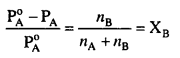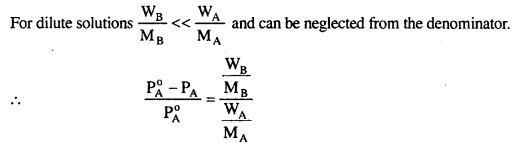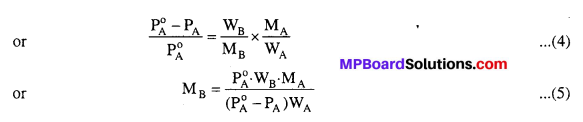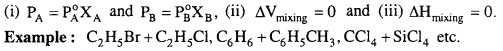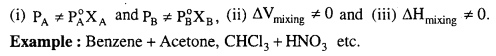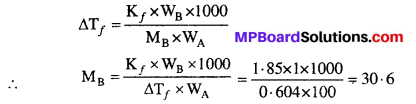MP Board Class 6th Science Solutions Chapter 2 Components of Food
Components of Food Textbook Exercises
Question 1.
Name the major nutrients in our food?
Answer:
The major nutrients, in our food are proteins, fats, carbohydrates, vitamins and minerals. In addition to above, food also contains dietary fibres and water.
Question 2.
Name the following:
- The nutrients which mainly give energy to our body.
- The nutrients that are needed for the growth and maintenance of our body.
- A vitamin required for maintaing good eyesight.
- A mineral that is required for keeping our bones healthy.
Answer:
- Fats and carbohydrates
- Proteins
- Vitamin A
- Calcium
![]()
Question 3.
Name two foods each rich in:
- Fats
- Starch
- Dietary fibre
- Protein.
Answer:
- Fats: Groundnuts, til, milk, ghee.
- Starch: Peanuts, dal (cooked), rice (cooked), raw potato.
- Dietary fibre: Fresh fruits, grains, pulses, potatoes.
- Protein: Gram, soyabeans, eggs, paneer.
Question 4.
Tick (✓) the statements that are correct?
- By eating rice alone, we can fulfill nutritional requirement of our body.
- Deficiency diseases can be prevented by eating a balanced diet.
- Balanced diet for the body should contain a variety of food items.
- Meat alone is sufficient to provide all nutrients to the body.
Answer:
- (x)
- (✓)
- (✓)
- (x)
![]()
Question 5.
Fill in the blanks:
- ……………………….. is caused by deficiency of Vitamin D.
- Deficiency of ………………………. causes a disease known as beri – beri.
- Deficiency of Vitamin C causes a disease known as ……………………….
- Night blindness is caused due to deficiency of ………………….. in our food.
Answer:
- Rickets
- Vitamin B
- Scurvy
- Vitamin A.
Projects And Activities
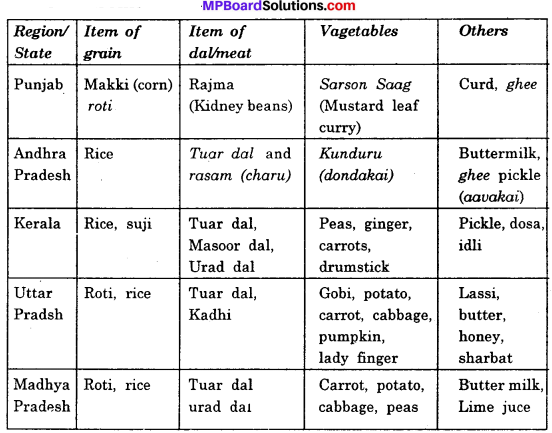
Activity 1.
Prepare a table to show that means from different regions/states?
Answer:
Some common meals of different regions/states
Activity 2.
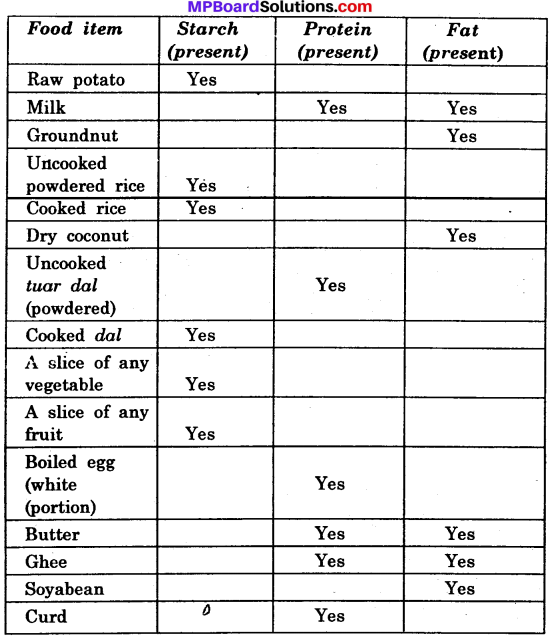
Prepare a table to show that various nutrients present in some food items?
Answer:
Nutrients present in some food items
Activity 3.
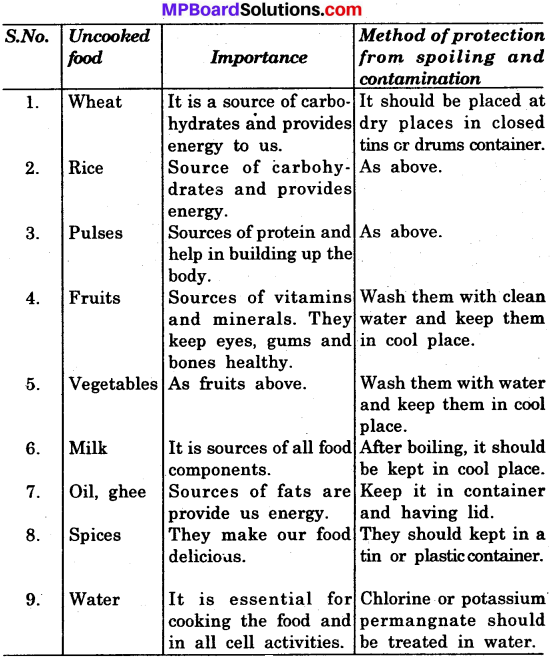
Make a list of uncooked items of food that are found around you. Indicate the importance of each one of these in your diet. How can these items be protected from spoiling or contamination?
Answer:
Uncooked food items, their importance in diet and method of protection.
Activity 4.
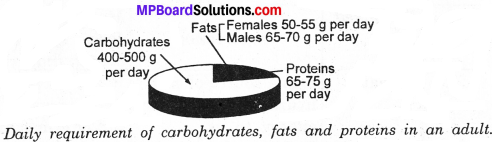
Define diagrammetically the daily requirement of carbohydrates, fats and proteins in an adult?
Answer:
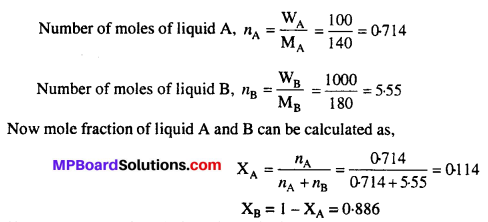
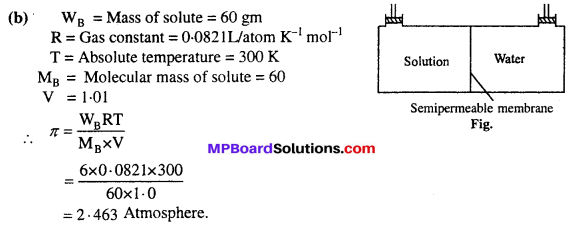
Daily requirement of carbohydrates of an adult is about 400 g to 500 g per day and that of proteins is 65 g to 75 g per day. The daily requirement of fats for females is 50 g to 55 g per day and for males 60 g to 70 g per day. During pregnancy and lactation period, protein requirement in females is greater than that for the males.
Activity 5.
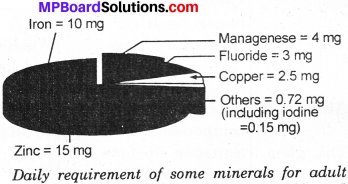
Define diagrametically the daily requirement of some minerals in an adult?
Answer:
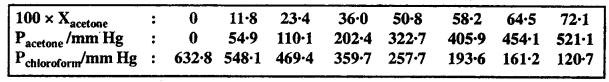
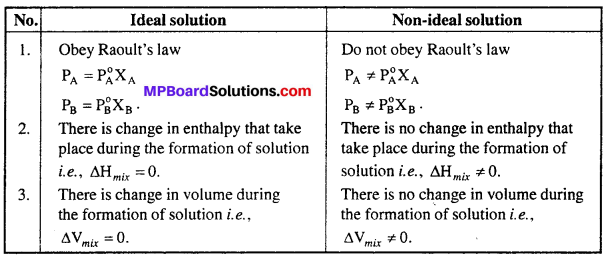
The minerals present in our body are mainly in the form of compounds of sodium phosphorous, calcium, chlorine, iron, potassium, sulphur, copper and iodine. Only small amount of minerals are required in our daily diet. Each one of these minerals is necessary for a proper growth of the body and to maintain good health. Following figure shows the daily requirement of some minerals for adults.
Activity 6.
Define diagrametically the daily requirements of some vitamins in adults?
Answer:
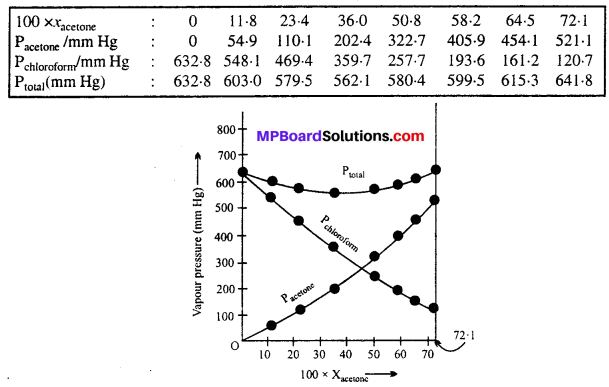
Vitamins are an essential component of our diet as they perform specific functions in our body. Different types of vitamins have been given specific names, like vitamin A, vitaminC, vitamin D, vitamin E and vitamin K. Some of the vitamins are soluble in water while some others dissolve only in fats. Daily requirement (in mg) of various vitamins are shown in following diagram: vitamin A = 0.5 mg
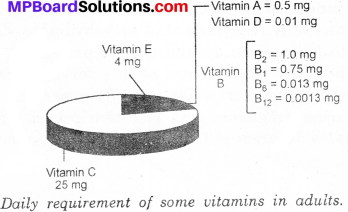
Activity 7.
What is the basic functions of food and what it does to our body?
Answer:
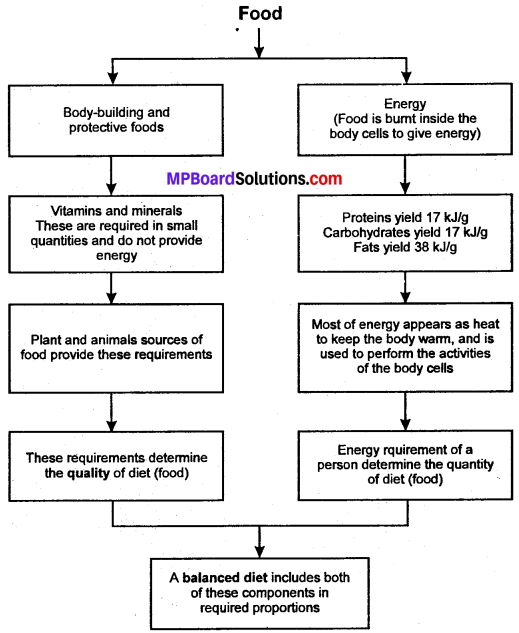
Components of Food Intex Questions
Question 1.
Our body also prepares Vitamin D in the presence of Sunlight?
Answer:
Yes.
Question 2.
Paheli wonders whether animal food also consists of these different components and do they also need a balanced diet?
Answer:
Yes, animals also need a balanced diet.
Components of Food Additional Important Questions
Objective type Questions Components of Food
Question 1.
Choose the correct answer:
Question (a)
Which one of the following foods provides energy to the body:
(a) carbohydrates
(b) proteins
(c) minerals
(d) vitamins.
Answer:
(a) carbohydrates
Question (b)
Salad in our diet mainly contains:
(a) carbohydrates
(b) fats
(c) proteins
(d) roughage.
Answer:
(d) roughage.
![]()
Question (c)
Which one of the following is an example of fats?
(a) Banana
(b) Wheat
(c) Butter
(d) Lemon.
Answer:
(c) Butter
Question (d)
Kishmish is a dry form of:
(a) grapes
(b) watermelon
(c) mango
(d) none of the above.
Answer:
(a) grapes
Question (e)
Which one of the following represents a balanced diet?
(a) Leafy vegetables
(b) Mango
(c) Milk
(d) Wheat and rice.
Answer:
(c) Milk
Question (f)
The total requirement of fats for an adult is about –
(a) 60 g to 80 g per day
(b) 50 g to 70 g per day
(c) 80 g to 100 g per day
(d) None of the above.
Answer:
(a) 60 g to 80 g per day
![]()
Question (g)
Communicable diseases are caused by:
(a) virsues
(b) bacteria
(c) fungi
(d) all of these.
Answer:
(d) all of these.
Question (h)
Which is a water – soluble vitamin?
(a) Vitamin A
(b) Vitamin C
(c) Vitamin D
(d) all of these.
Answer:
(b) Vitamin C
Question (i)
Beri – Beri is caused due to the deficiency of vitamin:
(a) A
(b) B
(c) D
(d) K
Answer:
(b) B
Question (j)
One of the following is not a communicable disease –
(a) malaria
(b) scurvy
(c) typhoid
(d) dysentery.
Answer:
(b) scurvy
![]()
Question 2.
Fill in the blanks:
- Organism feeding on flesh are called ………………………….
- Less intake of proteins in diet causes ………………………….
- Less intake of nutrients than required is …………………………..
- Carbohydrates and fats are composed of ………………………..
- Pulses are rich in …………………………..
- Starch is a ………………….. sugar.
- ………………….. is the essence of life.
- A …………………….. includes both of these components in required proportion.
- …………………….. is an example of saturated fat.
- A bad taste and fuel smell indicates that the food has been infested with ………………………..
- Vitamin K helps in ……………………….
- Deficiency of B12 causes ………………………..
- Deficiency of iron causes ………………………….
- ……………………….. and ……………………… are essential nutrients.
- Lack of vitamins causes and leads to ………………………..
Answer:
- Carnivorous
- Kwashiorker
- Malnutrition
- Carbon, hydrogen and oxygen
- Proteins
- Polymers
- Water
- Balance diet
- Vanaspati
- Micro – organisms,
- Clotting of blood
- Anaemia
- Anaemia
- Vitamins, minerals
- Specific diseases.
![]()
Question 3.
Which of the following statements are true (T) or false (F):
- Proteins supply the maximum calories to our bodies.
- We can live without proteins.
- A diet that supplies enough calories is a balanced diet.
- Protein is a staple food.
- Potato is rich in carbohydrates.
- Tomatoes contain Vitamin C.
- Milk, meat, pulses and fish are sources of proteins.
- Fats contain more energy than carbohydrates.
- Expensive food is not always the best food.
- Roughage contains all the food components.
- High dose of vitamins to children may not harmful.
- Rice is one of the major basic foods.
- Over – eating does not causes any diseases.
- Vitamin D deficiency may occur during pregnancy and lactation.
- Vitamin C is not soluble vitamin.
- Phosphorus is very important for the development of body.
- Deficiency of Vitamin A makes our bones weak.
- Deficiency of iron causes paleness.
- Deficiency of Vitamin D cause swollen and bleeding gums.
- Deficiency of vitamin B help to increase for palliate.
Answer:
- False
- False
- False
- False
- True
- True
- True
- True
- True
- False
- False
- True
- False
- True
- False
- True
- False
- True
- False
- False.
Question 4.
Match the items of Column A with the items of Column B:
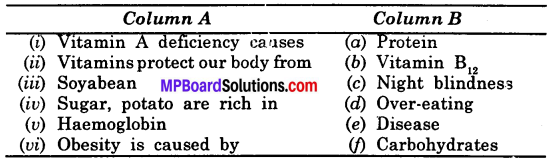
Answer:
(i) – (c)
(ii) – (e)
(iii) – (a)
(iv) – (f)
(v) – (b)
(vi) – (d)
Components of Food Very Short Answer Type Questions
Question 1.
Which of the following produce energy: Fat or Carbohydrates?
Answer:
Carbohydrates.
Question 2.
What is calorie?
Answer:
Calorie is the unit of heat. The food which we take is oxidised, in the presence of oxygen with the liberation of energy.
Question 3.
Which one offers you more energy 100g of grapes or one banana?
Answer:
One banana.
![]()
Question 4.
Which has more vitamins 100 g of grapes or 100g of spinach?
Answer:
100g of spinach.
Question 5.
What are nutrients?
Answer:
Nutrients are the components of food that the body needs in adequate amount for growth to reproduce and lead a normal healthy life.
Question 6.
Write the sources of car body drates?
Answer:
The carbohydrates are mainly found in sugar, wheat, maize and cereal etc.
Question 7.
What are the sources of fats?
Answer:
The sources of fats are ghee, butter, nuts and vegetable oils.
Question 8.
How much energy is produced from one gram of carbohydrates?
Answer:
16.8 kJ of energy is produced from one gram of carbohydrates.
Question 9.
Write the names of any two water soluble vitamins?
Answer:
Vitamin B and C.
![]()
Question 10.
What are the sources of Vitamin A?
Answer:
The sources of Vitamin A are milk, carrot, fish, oil etc.
Question 11.
Name the fat soluble Vitamins?
Answer:
Vitamin A and D.
Question 12.
What are the sources of Vitamin D?
Answer:
The sources of Vitamin D are eggs, fish, oil and milk products.
Question 13.
Which mineral is vital for bones and teeth?
Answer:
Calcium and phosphorus.
Question 14.
Name the main constituent of roughage?
Answer:
Cellulose is the main constituent of roughage.
Question 15.
What do you mean by staple food?
Answer:
The main food that we eat to provide us energy is called staple food. For example, chapati, rice, bread, etc.
Question 16.
Name the two biotic factors that damage the food – grains?
Answer:
- Temperature, and
- Moisture content.
![]()
Question 17.
Grapes get spoiled faster as compared to apples. Why?
Answer:
Because grapes contain more water content than apples, so they spoil faster.
Question 18.
Write the cause for food poisoning?
Answer:
Food poisoining is caused by micro – organisms like bacteria which can reproduce rapidly.
Question 19.
What is dehydration?
Answer:
Removal of water from fruits and vegetables is called dehydration.
Question 20.
Name two sources each of animal and vegetable proteins?
Answer:
Sources of animal proteins are Egg, Meat, Fish and Milk. Sources of vegetable proteins are Pulses, Peas, Bean and Soyabean.
Question 21.
What are the symptoms of Vitamin C deficiency?
Answer:
Deficiency of Vitamin C causes trouble in gums.
Question 22.
Name the disease caused by deficiency of Vitamin A?
Answer:
Deficiency of Vitamin A causes weakness in eyes and night blindness.
Question 23.
Same mass of which nutrient gives more energy fats or carbohydrates?
Answer:
The fats produce more energy than carbohydrates because they have less oxygen percentage. A gram of carbohydrate produce 4.2 kcal While a gram of fat produce 9.1 kcal of heat.
Question 24.
What is a balanced diet?
Answer:
A meal which contains various constituents of food which are necessary to keep the body healthy. A balanced meal has an appropriate food ratio of carbohydrate, protein, fat, vitamins and minerals.
![]()
Question 25.
Mention the factors which effect our health?
Answer:
The factors which effect our health are:
- Unbalanced food
- Diseases caused by infection.
Question 26.
What are the major factors affecting the human health?
Answer:
The major factors affecting the human health are:
- Intrinsic (or internal) factors
- Extrinsic (or external) factors.
Question 27.
Define intrinsic factors?
Answer:
The disease causing factors which exist within the humun body are called intrinsic factors?
Question 28.
Define extrinsic factors?
Answer:
The disease causing factors which come from outside the human body are called extrinsic factors.
Question 29.
Name the disease caused by deficiency of Vitamin C?
Answer:
Scurvy.
Question 30.
Name the disease caused by deficiency of Vitamin D?
Answer:
Rickets.
Question 31.
How much proteins do you need in your daily diet?
Answer:
We need proteins according to our body weight which is 2.5 gm per kilogram weight of the body.
![]()
Question 32.
When you fry your food in oil, which Vitamins are generally lost?
Answer:
Vitamin C.
Question 33.
Name some water – borne diseases?
Answer:
Water – borne disease are jaundice, cholera, polio, diarrhoea, typhoid.
Question 34.
Name some air – borne diseases?
Answer:
Air – borne diseases are whooping cough, common cold.
Question 35.
Name some of the diseases caused by extrinsic factors?
Answer:
Kwashiorkor, goitre, obesity, malaria, T.B., AIDS etc.
Question 36.
List some food – borne diseases?
Answer:
Some food – brone diseases are diarrhoea, dysentery and cholora during the rainy season.
Components of Food Short Answer Type Questions
Question 1.
How can we say that the fats are like an energy bank in living organism?
Answer:
We know that the fats have more calories of energy than carbohydrates in a unit mass. Fats have less oxygen and give more energy. Only fat can be stored for the future use as the polar bear does. It takes food before winter and then hibernates for several months. During this period the stored fat is consumed and thus fat acts as an energy bank.
Question 2.
How will you test for carbohydrate?
Answer:
To test the carbohydrate we take the given material and heat it with water. Then we put two drops of iodine solution in it and see the result. If the colour is changed to blue black, then carbohydrate is there otherwise not.
![]()
Question 3.
Name any three sources of carbohydrates?
Answer:
Carbohydrates are the compounds of carbon, hydrogen and oxygen. These are one of the compound of our food which provide us energy. Various sources from where we get carbohydrates are rice, wheat, cereals, sugar etc.
Question 4.
What are proteins?
Answer:
Proteins are the polymers of amino acids. There are only twenty amino acids known to us. They link together to form proteins. The amino acids are made up of carbon, hydrogen, oxygen and nitrogen. However some also contains phosphorous, sulphur etc. The important sources of proteins are meat, fish, egg, milk and all pulses.
Question 5.
What are minerals?
Answer:
Minerals are the chemical elements present in our food. They help to regulate various metabolic activities in our body. The importatant minerals required are calcium, phosphorous, iron, iodine, potassium sodium and magnesium. Calcium and phosphorous are required for bone and teeth formation, iron is required for the formation of haemoglobin and sodium and potassium are required for normal functioning of the nerve cells.
Question 6.
Why does living organisms required food?
Answer:
Every living being needs energy for its life processes. This energy can be obtained only from the food. So to meet out the energy requirement of the body, food is necessary. Food is also needed for growth and control of various life activities of the body.
![]()
Question 7.
What are the three important qualities of balanced diet?
Answer:
The three important qualities of balanced diet are as follows:
- It should be rich in essential nutrients such as vitamins, minerals, amino acids, etc.
- It should be able to provide enough raw material to meet the basic needs of growth, repair and replacement of cells in our body.
- It should provide energy required by the body.
Question 8.
How can you vary your diet without making it costlier?
Answer:
We can vary our diet by adopting the following instructions without making it costlier:
- We should take seasonal vegetables and fruits because they are cheap at that time.
- Rice and wheat should be eaten alternately so that a balanced diet may be obtained at low cost.
- We should use cheap and nutritious fruits e.g., banana, guava, are more nutritious than grapes.
Question 9.
Name the foods needed?
- For strong bones and teeth.
- To prevent scurvy.
- To avoid constipation.
- For warmth.
- For growth.
Answer:
- For strong bones and teeth. Milk, Fish, Oils, Eggs,
- To prevent scurvy. All citrus fruits, Amla, Orange, Lemon, etc.
- To avoid constipation. Water, Juicy fruits, Fresh vegetables, etc
- For warmth. Meat, Fish,
- For growth. Green leafy vegetables, Milk.
Question 10.
Explain why you should:
- Eat less cakes and ice – cream.
- Remove most of the fat from meat.
- Eat fresh food instead of processed food.
- Eat more fruits and vegetables.
Answer:
- Cakes and ice – cream have too much carbohydrates and sugar.
- Fat is not digested quickly. It is used as fuel in the deficiency of carbohydrates only otherwise in deposits in the inner side of blood vessels.
- The perishable food items are processed to keep them edible for a long time but many food nutrients are destroyed during processing, so we should eat fresh food. Moreover the nutrients present in the food can easily be processed food.
- Fruits and vegetables contain more food nutrients than the preserved and non-perishable food items, so we should eat more fruits and vegetables.
![]()
Question 11.
What is the main difference between vitamins and minerals?
Answer:
Vitamins:
- Vitamins are chemical substances which help the proteins particularly enzymes in their proper functioning.
- Their main source are fruits, vegetables, milk and other food items. They cannot be extracted from the earth.
Minerals:
- Minerals are the main constituents of our body parts such as teeth, bone blood etc.
- The minerals can be extracted from the earth. But animals obtain them from fruits, vegetables, milk, etc.
Question 12.
What are deficiency diseases?
Answer:
There are many vitamins which are used as nutrients. They are named by alphabet letters such as A, B, B1, B2, B6, B12, C, D, K. Each of them plays its own role. If any one of them is not present, an abnormality in the body can be seen. Such abnormalities are known as vitamin deficiency diseases.
Question 13.
List some diseases that are caused by vitamin deficiency in the body.
Answer:
Vitamin deficiency diseases are:
Components of Food Long Answer Type Questions
Question 1.
What are the various functions of protein?
Answer:
The various functions of proteins are:
- They form enzymes which are very important for living organisms.
- They are able to repair cells of the body which have undergone wear and tear.
- They are also used in making new cells.
- Proteins help in building of the body.
- Proteins help in digestion of body.
- Haemoglobin is a kind of protein which helps in the transportation of oxygen and carbon dioxide.
- Muscle, skin, hair and nails are all proteins.
- Proteins also act as the body materials.
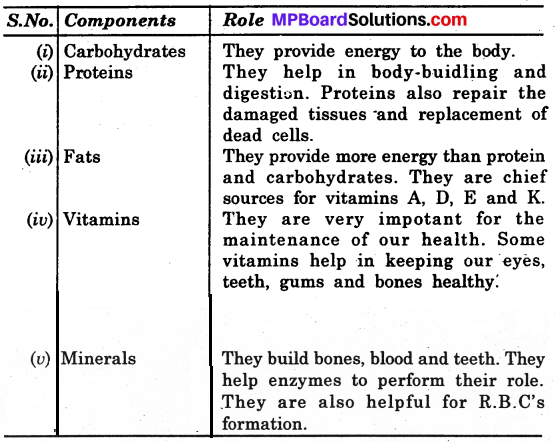
Question 2.
What are the roles of each of these components?
Answer:
Components of Food
- Carbohydrates
- Proteins
- Fats
- Vitamins
- Minerals.
Question 3.
How can you balance your diet without adding to its cost? Suggest any one method to do so?
Answer:
We should eat the things which are easily available and cheap and having the food nutrients in equal quantity as that of costly food items. We should find out the nutrients and their percentage in the edible things and also their cost and then suggest the people to eat those things.
A list is given for average daily calorie needs of people of different ages.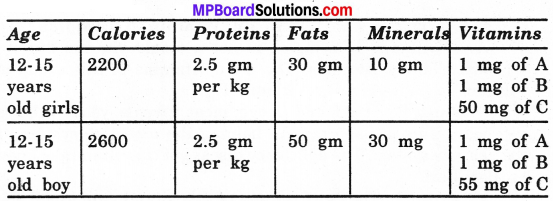
Question 4.
What are carbohydrates?
Answer:
The carbohydrates are components of carbon, hydrogen and oxygen. The simple carbohydrate is glycose. The other carbohydrates include sucrose, lactose, sugar, starch etc. The staple foods like rice, wheat, maize are rich sources of carbohydrates together with potato, sugarcane, grapes etc.
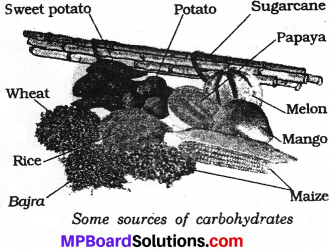
Question 5.
Draw a neat diagram of some sources of fats?
Answer:
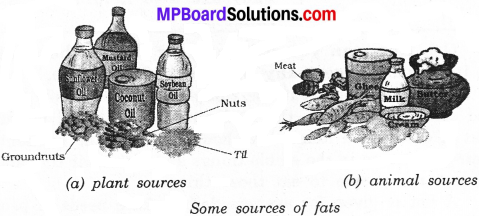
Question 6.
Write a short note on Vitamin B complex?
Answer:
Vitamin ‘B’ complex is not a single vitamin but it is a group of many vitamins. The main vitamins of this group are Vitamin B1, Vitamin B2, Vitamin B12.
Vitamin B1:
It is found in egg, meat, cereals, yeast, cabbage, soyabean. This is important in helping the digestive system and the nervous system. The deficiency disease of this vitamin is known as Beri – Beri.
Vitamin B2:
The chief sources of this vitamin are green leafy vegetables, peas, beans, cheese. It is helpful in keeping our mouth and skin healthy and for normal growth.
Vitamin B12:
This vitamin is available in milk, cheese, etc. and is responsible for proper growth of the body. The deficiency disease due to this vitamin is the anaemia.
Question 7.
Write down the sources, importance and deficiency diseases of Vitamin A, Vitamin C, Vitamin D and Vitamin K?
Answer:
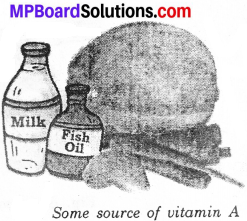
Vitamin A:
The main sources of this vitamin are milk, butter, cheese, egg, liver oils, green and yellow vegetables. This is important for eyes, hair and skin and the deficiency disease due to Vitamin ‘A’ is night blindness.
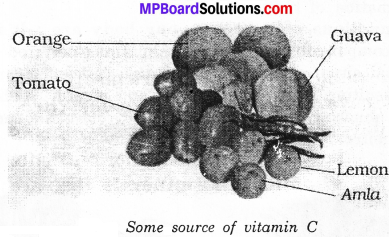
Vitamin C:
Citrus fruits as the lemon, organges are the main sources and is also available in plenty amount in goose berries, guava and amla. This vitamin is helpful in keeping teeth, gums and joints healthy. The deficiency disease is known as the scurvy.
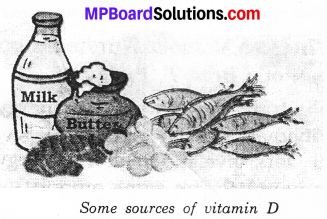
Vitamin D:
The main sources are fish, liver oil, milk. Our body can prepare it in sunlight. It is essential for the normal growth of the bones. The deficiency disease is known as the rickets.
Vitamin K:
It is available in green leafy vegetables, tomatoes and egg yolks in sufficient amount. Due to its deficiency there is excessive bleeding after injury. Vitamin D helps in clotting of the blood.
Question 8.
What are the functions of iron and iodine in the body?
Answer:
The function of iron in our body are:
It gives red colour to the blood and transmits oxygen to body. It is needed for the formation of blood cells (RBC). The functions of iodine in our body are: It is very important component of thyroxin, a hormone secreted by the thyroid gland situated in the neck. Iodine deficiency can cause disorders resulting in retarded growth and mental disability. It also causes abnormal enlargment of the thyroid gland commonly known as goitre.