MP Board Class 12th Biology Important Questions Chapter 16 Environmental Issues
Environmental Issues Important Questions
Environmental Issues Objective Type Questions
Question 1.
Choose the correct answer:
Question 1.
Disease caused due to mercury pollution is:
(a) Methenamine
(b) Minimata
(c) Asbestosis
(d) Liver inflammation.
Answer:
(b) Minimata
Question 2.
Plants are natural air purifiers because they exhibit :
(a) Respiration
(b) Photosynthesis
(c) Transpiration
(d) Drying.
Answer:
(b) Photosynthesis
Question 3.
Metal found in atmosphere is :
(a) Cadmium
(b) Lead
(c) Mercury
(d) Zinc.
Answer:
(b) Lead
![]()
Question 4.
Burning of fossil fuel results in:
(a) SO2 pollution
(b) NO2 pollution
(c) N2O pollution
(d) NO pollution.
Answer:
(a) SO2 pollution
Question 5.
Increase of the concentration of which gas results greenhouse effect :
(a) CO2
(b) CO
(c) O2
(d) Nitrogen oxide.
Answer:
(a) CO2
Question 6.
Gas responsible for depletion of O3 in atmosphere is :
(a) CFC
(b) NO2
(c) CO2
(d) SO2
Answer:
(a) CFC
Question 7.
Carbon monoxide is the chief pollutant of :
(a) Water
(b) Air
(c) Noise
(d) Soil.
Answer:
(b) Air
Question 8.
Effect of air pollution is seen in :
(a) Leaves
(b) Flower
(c) Forests
(d) None of these.
Answer:
(b) Flower
Question 9.
Shrinking of leaves occurs by :
(a) SO2
(b) O2
(C) H2S
(d) CO.
Answer:
(a) SO2
Question 10.
Example of greenhouse gas is :
(a) CO2
(b) CH4
(c) CFC
(d) All of these.
Answer:
(d) All of these.
Question 11.
Gas produced during combustion of fossil fuels is :
(a) CO2
(b) CH4
(c) CFC
(d) N2O
(a) CO2
Question 12.
Gas used in refrigeration is :
(a) Freon
(b) N2O
(c) CH4
(d) CO2.
Answer:
(a) Freon
![]()
Question 13.
Radiation emitted due to heating of earth is:
(a) UV
(b) Infrared
(c) Gamma
(d) None of these.
Answer:
(b) Infrared
Question 14.
Gas responsible for depletion of atmospheric ozone layer is:
(a) CO2
(b) CH4
(c) CFC
(d) O2
Answer:
(c) CFC
Question 2.
Fill in the blanks:
- ………………….. is a chief green house gas.
- ………………….. is the measurement unit of water pollution.
- ………………….. and ………………….. gases are responsible for acid rain.
- ………………….. gas is essential for life. (MP 2009 Set B)
- The substances which produce pollution are called ………………….. (MP 2013)
- Strong, loud, unpleasant or untolerable sound is called ………………….. (MP 2013)
- We can ………………….. the quantity of CO2 from forestation.
- CO2, CH4, N2O, CFCs are………………….. gases.
- ………………….. is discharged in Bhopal gas disaster.
- In human DDT is reached by …………………..
- ………………….. is also known as chemical weeds.
- Pollution is the …………………. changes which occurs in atmosphere.
- Deficiency of soil nutrients is called …………………..
- The percentage of CO2 of atmosphere can be ………………….. by forestation. (MP 2009 Set D)
Answer:
- CO2
- BOD
- SO2 and NO2
- O2
- Pollutant
- Noise
- Lost
- Green house
- Methyl isocyanate
- Food chain
- Ozone
- Unwanted
- Negative pollution
- Reduced.
Question 3.
Match the followings:
I.

Answer:
- (c)
- (f)
- (e)
- (b)
- (d)
- (a)
- (g)
- (h)
II.

Answer:
- (b)
- (c)
- (d)
- (a)
Question 4.
Write the answer in one word/sentences:
- Write full name of BOD.
- What is the hearing capacity of man?
- Name the fish used to control mosquito.
- What is the concentration of CO2 in environment?
- When is World Environment Day celebrated?
- Write the names of two main gases which pollute air.
- Write the name of highly polluted river of India.
- Name the term used, for the increase in atmospheric temperature, due to increase in CO2 concentration.
- What is the amount of oxygen taken up by microorganisms, that decompose waste matter?
- Write full name of CFC.
- Write the name of a chief greenhouse gas.
- Write the name of gas responsible for ozone layer depletion.
- Who is the greatest enemy of nature and organism? (MP 2013)
- When we celebrated ozone day?
Answer:
- Biological Oxygen Demand
- 10 – 12 decibel
- Gambussia
- 0 03%
- 5th June
- SO2and CO
- Ganga
- Green House effect
- B. O. D
- Chlorof – luorocarbon
- CO2
- CFC
- Global warming
- 16December.
Environmental Issues Very Short Answer Type Questions
Question 1.
What is the definition of pollution?
Answer:
Pollution is defined as an undesirable changes in physical, chemical or biological characteristics of air, land, wafer and soil.
Question 2.
What are the essential components of biodiversity?
Answer:
Essential components of biodiversity are:
- Genetic diversity.
- Species diversity.
- Ecological diversity.
Question 3.
When and how photochemical smog is formed?
Answer:
Photochemical smog is the chemical reaction of sunlight, nitrogen oxides and volatile organic compounds in the atmosphere, which leaves air borne particles and ground level ozone. Peroxyacetyl Nitrate (PAN) is present in the photochemical smog. It causes damage to respiratory system and environment system. (The word smog is derived from smoke and fog.)
![]()
Question 4.
Name two plants which uses carbon monoxide (CO).
Answer:
Two plants which uses CO are:
- Daucus carota,
- Ficus variegata.
Question 5.
Name the types of water pollution based on source of water.
Answer:
Types of water pollution based on source of water:
- Underground water pollution
- Surface water pollution
- Lake water pollution and
- River water pollution.
Question 6.
How does fertilizers pollute water?
Answer:
Excess fertilizers used in the field are drained into the pond and river, where they proliferate growth of algae, which spread all over the surface of the water and cut off oxygen supply for lower aquatic organisms. Due which lower aquatic organisms dies. This is known as eutrophication.
Question 7.
Give harmful effects of cutting of forest.
Answer:
Harmful effects of cutting of forest are:
- It increases landscape.
- It decreases fertility of the soil.
- Rainfall reduces and become uncontrolled.
Environmental Issues Short Answer Type Questions
Question 1.
What is acid rain? Write any two effects of acid rain on human beings.
Answer:
Acid rain:
SO2 gas is released into the environment by burning of fossil fuels containing sulphide from industries, example Coal. In presence of moisture, SO2 reacts with water and forms the droplets of sulphurous and sulphuric acid in the environment. Likewise nitrogen oxides released from motor vehicles, burning materials and chemical industries also react with water to produce the droplets of nitric acid. The droplets of sulphurous, sulphuric acid and nitric acids fall down on the earth with rainwater and thus, it is known as acid rain.
- SO2 + \(\frac { 1 }{ 2 }\) O2 → SO3
- H2O + SO3 → H2SO4
- H2O + NO2 → HNO3
Effects on human beings : Skin diseases, irritation, metabolic diseases.
Question 2.
Write the effect of air pollution on plants.
Answer:
Effect of air pollution on plants:
- Increase in the concentration of SO2 causes chlorosis of leaves in plants.
- The cells and chlorophyll of leaves are degraded and fall down.
- Vegetative and reproductive growth is inhibited.
- The development of plant is inhibited.
Question 3.
Write short note on pollution caused due to combustion activities.
Answer:
Combustion of fuel, such as wood, coal, natural gas for cooking and for some other puiposes gives out gases like CO2, CO, SO2 and consumes oxygen from the air.
![]()
Question 4.
Write preventive measures to control air pollution.
Answer:
Preventive measures to control air pollution:
- Industrial smokes must be filtered before releasing it into the atmosphere.
- Tree plantation should be increased and deforestation prevented.
- Use of automobiles should be minimized which reduce the nitrogen contents in the atmosphere.
- The use of crude fuels should be avoided and use of high quality fuels should be recommended.
- Nuclear explosions should be avoided.
- Legal control of air pollution.
- Plantation of air purifying plants.
- Development of parks and gardens in cities.
Question 5.
Write any four measures to control greenhouse effect.
Answer:
Following important measures can be sited as the base steps towards controlling greenhouse effect:
- The aim is achieved to some extent by reducing the consumption of fossil fuels such as coal and petroleum. This can be achieved by depending more on non – conventional renewable sources of energy such as solar, wind, tidal, biogas and nuclear energies.
- Disposing of the greenhouse gases as they are formed elsewhere than in the atmosphere.
- By recovering greenhouse gases present already in the atmosphere and disposing them off elsewhere.
- Prevention of deforestation and planting of more trees.
Question 6.
Write short note:
- Bio – magnification.
- UV rays.
- Biodegradable pollutants.
- Non – biodegradable.
Answer:
1. Bio – magnification:
Bio – magnification, also known as bio-amplification or biological magnification is the increasing concentration of a substance, such as a toxic chemical, in the tissues of organisms at successively higher levels in a food chain.
2. UV rays:
Sunlight is the chief source of ultraviolet rays. The rays of sunlight having a wavelength range from 200 to 390 nm are called as UV rays.
Effect of UV rays:
They have a direct effect on living cells. DNA and other chemical substances in cells are inactivated due to UV rays. Prevention of replication of the DNA molecule and its distortion cause many ill effects.
3. Biodegradable pollutants:
The pollutants which are degraded by natural factors and decomposed by natural activities are known as biodegradable pollutants, e.g., Domestic sewage, heat. The domestic sewage can be rapidly decomposed by natural processes or in engineered system (sewage treatment plant) that enhance natures great capacity to decompose and recycle.
4. Non – biodegradable:
The pollutants which cannot be purified by natural methods are called non-biodegradable pollutants. Plastic products, many chemicals, DDT, long chain detergents, glass aluminium, mercury salt and other synthetic products manufactured by man come under this category. These non – biodegradable pollutants not only accumulate but often “biologically magnified” as they move in biochemical cycles and along food chains.
Question 7.
What is the effect of SO2 in the environment?
Answer:
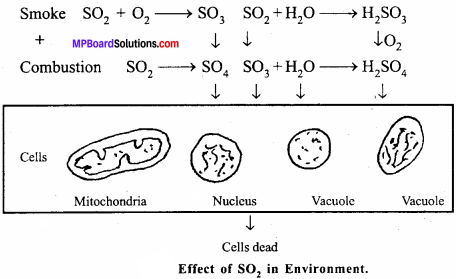
Question 8.
Write only the sources of water and air pollution.
Answer:
1. Sources of water pollution : Following are the chief sources of water pollution:
(a) Human sources:
- Domestic sewage
- Industrial effluents
- Agricultural wastes
- Oil pollution
- Thermal and nuclear power station.
(b) Natural sources:
Water pollution takes place by natural ways like soil erosion, mixing of metallic substances, plant leaves, humus and faecal matter of animals etc.
2. Sources of air pollution : Following are the chief sources of air pollution:
(a) Human sources:
- Combustion activities
- Industrial activities
- Agricultural works
- Use of solvents
- Activities concerned with atomic energy.
(b) Natural sources:
Volcano and its lava, ash, dust, smoke of forest fire, winds, cyclone. Decomposition of matters in the swamp water and liberation of methane gas and different compounds of hydrogen from forests plants, various pollen grains etc.
Question 9.
Write a note on the effect of water pollution on aquatic organisms.
Answer:
Effect of water pollution on aquatic plants:
1. Inorganic nitrates and phosphates in excess amount stimulates excessive plant growth in lakes and reservoirs. These plants deplete the oxygen contents of the water during night. This leads to suffocation of fish and other aquatic life.
2. The rapid algal growth leads to the diminishing of nutrient in the medium causing rapid decay of algal filament. This increases productivity of lake and stream water brought about by nutrient enrichment is called eutrophication.
3. The number of microorganisms increases in polluted water.
4. Siltation occurs in water.
5. Temperature of water increases and O2 ratio is reduced.
Effect of water pollution on aquatic animals : Animal life depends on aquatic plants. Aquatic animals are affected from water pollution as follows:
- Decrease in the value of B.O.D. resulting in the number of aquatic animals.
- Animals found in freshwater and killed.
- Animal diversity is also decreased and fishes suffer from various types of diseases.
- Terrestrial animals are also affected by the use of polluted water.
Question 10.
Write the measures for control and prevention of water pollution.
Answer:
Measures for control and prevention of water pollution are:
Preventive measures:
- Use of harmful pesticides and weedicides must be stopped completely.
- Discharge of effluents into rivers, lakes and sea should be strictly prohibited without treatment.
- Oil spill should be prevented.
- Proper disposal of sewage so, that it does not find its way into water bodies.
- Preventing bathing, washing cloths, throwing dead bodies and other wastes into water source.
Curative measures:
1. Adequate waste water treatment:
The domestic sewage and the industrial waste should be properly treated before its disposal into water ways.
Waste water treatment involves three steps :
(a) Primary treatment – During this treatment large objects and suspended undissolved solids are removed and converted into the sludge, a valuable fertilizer.
(b) Secondary treatment – Aeration is supplied to promote bacterial decomposition, followed by chlorination to reduce its content of bacteria.
(c) Tertiary treatment – During this phase nitrates and phosphates are removed. The treated water is then released. Sewage treatment is quite expensive and in many developing countries only the first two steps are followed.
2. Treatment of industrial effluents:
Industrial effluents should be properly treated to remove the pollutants. These involve neutralization of acids and alkalies, removal of harmful chemicals, coagulation of colloidal impurities, precipitation of metallic compounds and reducing the temperature of wastes to decrease thermal pollution. Chemical oxidation can be achieved by chlorination through reaction with ozone. However, there are certain chemicals which are difficult to remove.
3. Recycling:
One of the best methods of prevention and control of water pollution is the recycling of the various kinds of pollutants and wastes, example Dung of cow and buffalo can be used for the production of gobar gas a cheap source of fuel and also as manure.
![]()
Question 11.
Write short notes on air pollution caused due to industries.
Answer:
Industrial pollution is caused by industrial pollutants which are released by the industries such as SO2, CO2, CO, H2S and hydrocarbons together with dust and smoke. These are produced by the burning of coal and petroleum. The chemical industries releases HC1, Cl2, nitrogen oxide, oxides of copper, zinc, lead, arsenic etc. Industrial pollutants causes air pollution and water pollution.
Question 12.
Write the effects of noise pollution.
Answer:
Effects of Noise pollution:
- The more acute and immediate effect of noise pollution is impairing of hearing leading to auditory fatigue and may finally lead to. deafness.
- Interference with speech communication.
- Noise pollution leads to neurosis, anxiety hypertension, cardiovascular disease, hepatic stress, giddiness.
- Annoyance leading to ill – temper, bickering, mental disorientation and violent behaviour.
- The high intensity of noise pollution can cause blood vessels to contract, skin becomes pale, muscles to contract and adrenaline to be short into blood stream with consequence rise in blood pressure. This ultimately results in tension and nervousness.
- Affects different metabolic activities.
Question 13.
What are the various constituents of domestic sewage? Discuss the effects of sewage discharge on a river.
Answer:
Domestic sewage are the waste originating from the kitchen, toilet, laundry and other sources. They contain impurities such as suspended solid (sand, salt, clay), colloidal materials (faecal matters, bacteria, plastic and cloth fiber), dissolved materials (nitrate, phosphate, calcium, sodium, ammonia) and disease – causing microbes.
When organic wastes from the sewage enter the water bodies, they serve as a food source for microorganisms such as algae and bacteria. As a result, the population of these microorganisms in the water body increases. Here, they utilize most of the dissolved oxygen. for their metabolism. This results in an increase in the levels of BOD in river water and results in the death of aquatic organisms. Also, the nutrients in the water lead to the growth of planktonic algal, causing algal bloom. This causes deterioration of water quality and fish mortality.
Question 14.
List all the wastes that you generate at home, school or during your trips to other places, could you very easily reduce. Which would be difficult or rather impossible to reduce?
Answer:
Wastes generated at home include plastic bags, paper napkin, toiletries, kitchen wastes (such as peelings of vegetables and fruits, tea leaves), domestic sewage, glass, etc. Wastes generated at school include waste paper, plastics, vegetable and fruit peels, food wrapping, sewage, etc.
Wastes generated at trips or picnics include plastic, paper, vegetable and fruit peels, disposable cups, plates, spoons etc. Yes, wastes can be easily reduced by the judicious use of the above materials. Wastage of paper can be minimized by writing on both sides of the paper and by using recycled paper.
Plastic and glass waste can also be reduced by recycling and re-using. Also, substituting plastics bags with biodegradable jute bags can reduce wastes generated at home, school or during trips. Domestic sewage can be reduced by optimizing the use of water while bathing, cooking, and other household activities. Non – biodegradable wastes such as plastic, metal, broken glass, etc. are difficult to decompose because microorganisms do not have the ability to decompose them.
Question 15.
Write preventive measures of sound pollution.
Answer:
Preventive measures of noise pollution:
- Noise pollution can be controlled by designing quieter machines, proper lubrication and better maintenance of machines. Noise producing parts of machines may be covered by suitable insulating materials.
- Noise producing industries should be installed away from residential areas.
- Use of public addressing systems should be restricted at a fixed intensity and hours of the day.
- Stuffing of cotton plugs in the ear or use of earmuffs and minimise the danger of occupational exposure of noise.
- Trees absorb sound vibrations and thus, reduce the noise pollution. Thus, plantation of trees along highways, establishment of parks help in reducing noise pollution.
- By use of silencer in all automobiles and engines.
Question 16.
How many types of pollution are there? Explain in brief.
Answer:
Pollution are of following five types:
- Air pollution
- Noise pollution
- Water pollution
- Radioactive pollution
- Soil pollution.
1. Air pollution – Pyrotechnics results in the release of harmful gases like CO2, CO and SO2 in the environment which produces various types of diseases in living beings.
2. Noise pollution – Pyrotechnics produces the unwanted sound dumped into the atmosphere leading to health hazards.
3. Water pollution – Pyrotechnics produces some waste materials, on reaching water it causes water pollution.
4. Radioactive pollution – Pollution created due to radioactive substance is called radioactive pollution. Atomic energy centres and bombarding also causes radioactive pollution.
5. Soil pollution – Any change in physical and chemical characteristics of soil due to addition of unwanted substances which adversely affects productivity of soil is termed as soil pollution.
![]()
Question 17.
Discuss the role of women and communities in protection and conservation of forests.
Answer:
Women and Communities have played a major role in environmental conservation movements. The Bishnoi community in Rajasthan strictly believes in the concept of living peacefully with nature. In 1731, the King of Jodhpur ordered his ministers to arrange wood for the construction of his new palace. For this purpose, the minister and the workers went to bishnoi village. There, a Bishnoi woman called Amrita Devi along with her daughter and hundreds of other Bishnoi’s showed the courage to step forward and stop them from cutting trees.
They embraced the trees and lost their lives at the hands of soldiers of the king. This resistance by the people of the village forced the king to give up the idea of cutting trees. The Chipko movement was started in 1974, in the Garhwal region of the Himalayas. In this movement, the women from the village stopped the contractors from cutting forest trees by embracing them.
Question 18.
What measures, as an individual you would take to reduce environmental pollution?
Answer:
The following initiatives can be taken to prevent environmental pollution:
Measures for preventing air pollution:
- Planting more trees.
- Use of clean and renewable energy sources such as CNG and Bio – fuels.
- Reducing the use of fossil fuels.
- Use of catalytic converters in automobiles.
Measures for preventing water pollution:
- Optimizing the use of water.
- Using kitchen waste water in gardening and other household purposes measures for controlling noise pollution :
Avoid burning crackers on Diwali , Plantation of more trees.
Measures for decreasing solid waste generation:
- Segregation of waste.
- Recycling and reuse of plastic and paper.
- Composting of biodegrable kitchen waste.
- Reducing the use of plastics.
Question 19.
What are the causes of air pollution?
Answer:
The causes of air pollution:
The main causes of air pollutions are fossil fuels industries, factories and particulate matter are produced due to combustion of petrol, diesel, kerosene etc. in vehicles, houses and factories which pollute air. In thermal power plants, steel and glass industries, paper and sugar mills etc. combustion of coal and furnace oil produce carbon monoxide, carbon dioxide, sulphur dioxide, ash, dust particles and some heavy metals which are released into atmosphere. Similarly various types of chemicals released from cloth mills, cement industries, asbesters industries, pesticides industries etc. also causes air pollution.
Environmental Issues Long Answer Type Questions
Question 1.
Discuss the causes and effects of global warming. What measures need to be taken to control global warming?
Answer:
Global warming:
Global warming is defined as an increase in the average temperature of the Earth’s surface due to greenhouse effect.
Causes of global warming:
Global warming occurs as a result of the increased concentration of greenhouse gases in the atmosphere. Greenhouse gases include carbon dioxide, methane and water vapour. These gases trap solar radiation released back by the earth. Global warming is a result of industrialization, burning of fossil fuels, and deforestation.
Effects of global warming:
Global warming is defined as an increase in the average temperature of the Earth’s surface. It has been observed that in the past three decades, the average temperature of the Earth has increased by 0-6°C. As a result, the natural water cycle has been disturbed resulting in changes in the pattern of rainfall. It also changes the amount of rainwater. Also, it results in the melting of polar ice caps and mountain glaciers, which has caused a rise in the sea level, leading to the inundation of coastal regions.
Control measures for preventing global wanning:
- Reducing the use of fossil fuels.
- Use of bio – fuels.
- Improving energy efficiency.
- Use of renewable source of energy such as CNG, etc.
- Reforestation.
- Recycling of materials.
Question 2.
Write critical notes on the following:
- Eutrophication,
- Biological magnification,
- Groundwater depletion and ways for its replenishment.
Answer:
1. Eutrophication:
It is the natural ageing process of lake caused due to nutrient enrichment. It is brought down by the runoff of nutrients such as animal wastes, fertilizers, and sewage from land which leads to an increased fertility of the lake. As a result, it causes a tremendous increase in the primary productivity of the ecosystem. This leads to an increased growth of algae, resulting into algal blooms. Later, the decomposition of theses algae depletes the supply of oxygen, leading to the death of other aquatic animal life.
2. Biological magnification:
The increase in concentration of harmful non – biodegradable substances into higher tropic level is called biological magnification. DDT used to protect the crops reach the soil and are absorbed by plants with water and minerals from the soil. Due to rain, these chemicals can also enter water sources and into the body of aquatic plants and animals.
As a result, chemicals enter the food chain. Since, these chemicals cannot be decomposed, they keep on accumulating at each trophic level. The maximum concentration is accumulated at the top carnivore’s level. The producers (phytoplankton) were found to have 0 04 ppm concentration of DDT.
Since many, types of phytoplankton were eaten by zooplankton (consumers), the concentration of DDT in the bodies of zooplankton was found to be 0.23ppm. Small fish that feed on zooplankton accumulate more DDT in their body. Thus, large fish (top carnivore) that feed on several small fish have the have the highest concentration of DDT.
3. Groundwater depletion and ways for its replenishment:
The level of ground water has decreased in the recent years. The source of water supply is rapidly diminishing each year because of an increase in the population and water pollution. To meet the demand of water, water is withdrawn from water bodies such as ponds, rivers, etc. As a result, the source of groundwater is depleting.
This is because the amount or groundwater being drawn for human use is more than the amount replaced by rainfall. Lack of vegetation cover also results in very small amounts of water seeping through the ground. An increase in water pollution is another factor that has reduced the availability of groundwater.
Measures for replenishing groundwater:
- Preventing over-exploitation of groundwater.
- Optimizing water use and reducing water demand.
- Rainwater harvesting.
- Preventing deforestation and plantation of more trees.
![]()
Question 3.
Why ozone hole forms over Antarctica? How will enhanced ultraviolet radiations affect us?
Answer:
The ozone hole is more prominent over the region of Antarctica. It is formed due to an increased concentration of chlorine in the atmosphere. Chlorine is mainly released from Chlorofluorocarbons (CFC’s) widely used as refrigerants. The CFC’s release chlorine atoms by the action of UV rays on them. The release of chlorine atoms causes the conversion of ozone into molecular oxygen. One atom of chlorine can destroy around 10,000 molecules of ozone and causes ozone depletion.
- CF2Cl2 → CF2Cl + Cl
- Cl + O3 → ClO + O2
- ClO + O → Cl + O2
The formation of the ozone hole will result in an increased concentration of UV – B radiation on the Earth’s surface. UV – B damages DNA and activates the process of skin ageing. It also causes skin darkening and skin cancer. High levels of UV – B cause corneal cataract in human beings.
Question 4.
Discuss briefly the following:
- Radioactive wastes,
- Defunct ships and e-wastes,
- Municipal solid wastes.
Answer:
1. Radioactive wastes:
Radioactive wastes are generated during the process of ‘ generating nuclear energy from radioactive materials. Nuclear waste is rich in radioactive materials that generate large quantities of ionizing radiation such as gamma rays. These rays cause mutation in organisms which often results in skin cancer. At high dosage, these rays can be lethal. Safe disposal of radioactive wastes is a big challenge. It is recommended that nuclear wastes should be stored after pre-treatment in suitable shielded containers, which should then be buried in rocks.
2. Defunct ships and e – wastes:
Defunct ships are dead ships that are no longer in use. Such ships are broken down for scrap metal in countries such as India and Pakistan. These ships are a source of various toxicants such as asbestos, lead, mercury etc. Thus, they contribute to solid wastes that are hazardous to health.
E – wastes or electronic wastes generally include electronic goods such as computers, etc. Such wastes are rich in metals such as copper, iron, silicon, gold, etc. These metals are highly toxic and pose serious health hazards. People of developing countries are involved in the recycling process of these metals and therefore, get exposed to toxic substances present in these wastes.
3. Municipal solid wastes:
Municipal solid wastes are generated from schools, offices, homes and stores. It is generally rich in glass, metal, paper waste, food, rubber, leather and textiles. The open dumps of municipal wastes serve as a breeding ground for flies, mosquitoes and other disease causing microbes. Hence, it is necessary to dispose municipal solid waste properly to prevent the spreading of diseases. Sanitary landfills and incineration are the methods for the safe disposal of solid wastes.
Question 5.
What initiatives were taken for reducing vehicular air pollution.in Delhi? Has air quality improved in Delhi?
Answer:
Delhi has been categorized as the fourth most polluted city of the world in a list of 41 cities. Burning of fossil fuels has added to the pollution of air in Delhi. Various steps have been taken to improve the qualify of air in Delhi
Introduction of CNG (Compressed Natural Gas):
By the order of the Supreme Court of India, CNG powered vehicles were introduced at the end of year 2006 to reduce the levels of pollution in Delhi. CNG is a clean fuel that produces very little unbumt particles.
- Phasing out of old vehicles
- Use of unleaded petrol.
- Use of low – sulphur petrol and diesel.
- Use of catalytic converters.
- Application of stringent pollution-level norms for vehicles.
- Implementation of Bharat stage I, which is equivalent to euro II norms in vehicles of major Indian cities.
The introduction of CNG – powered vehicles has improved Delhi’s air quality, which has lead to a substantial fall in the level of CO2 and SO2. However, the problem of Suspended Particulate Matter (SPM) and Respiratory Suspended Particulate Matter (RSPM) still persists.
Question 6.
Discuss briefly the following:
- Greenhouse gases
- Catalytic converter
- Ultraviolet – B
Answer:
1. Greenhouse gases:
The greenhouse effect refers to an overall increases in the average temperature of the earth due to the presence of greenhouse gases. Greenhouse gases mainly consist of carbon dioxide, methane and water vapour. When solar radiations reach the Earth, some of these radiations are absorbed.
These absorbed radiations are released back into the atmosphere. These radiations are trapped by the greenhouse gases present in the atmosphere. This helps in keeping our planet warm and thus, helps in human survival. However an increase in the amount of greenhouse gases can lead to an excessive increase in the Earth’s temperature, thereby causing global warming.
2. Catalytic converter:
Catalytic converters are devices fitted in automobiles to reduce vehicular pollution. These devices contain expensive metals such as platinum, palladium and rhodium that act as catalysts. As the vehicular discharge passes through the catalytic converter, the unbumt hydrocarbons present in it get converted into carbon dioxide and water. Carbon monoxide and nitric oxide released by catalytic converters are converted into carbon dioxide and nitrogen gas (respectively).
3. Ultraviolet – B:
Ultraviolet – B is an electromagnetic radiation which has a shorter wavelength than visible light. It is a harmful radiation that comes from sunlight and penetrates through the ozone hole on to the earth’s surface. It induces many health hazards in humAnswer: UV – B damages DNA and activates the process of skin ageing. It also causes skin darkening and skin cancer. High levels of UV – B cause comeal cataract in human beings.
![]()
Question 7.
Describe the diseases caused due to radioactive pollution.
Answer:
Following diseases are caused by radioactive pollution:
- Leukaemia and Bone cancer – In human beings and other animals, radioactive pollution causes blood and bone cancer.
- Ageing – The reproductive capacity of organisms is decreased due to radioactive pollution and ultimately causes ageing.
- Epidemic disease – Radioactive pollution causes the decreased rate of antitoxin production which affects the immune system and results in the production of epidemic diseases.
- Nervous system and sensory cells become irritated.
- Radioactive pollution results skin cancer.
- Mutations also take place due to radioactive pollution.
- Thyroid cancer is also produced due to radioactive iodine.
Question 8.
Burning of crackers spread pollution in the environment. Explain.
Answer:
Explosive substances are used in crackers. Crackers are burned for celebration but it causes many harms to the living organisms. Some of the harm effects of burning of crackers are as follows:
1. Air pollution – It releases harmful gases such as CO2, CO, SO2 etc. into the atmosphere which causes respiratory diseases.
2. Noise pollution – Burning of crackers produces noise which causes noise pollution which effects our hearing capacity, causes headache, increases heartbeat and blood pressure.
3. Water pollution – Waste of burning of crackers are drained into the pond, river with rainwater or through drain which caused harm to aquatic organisms.
4. Soil pollution – Burning of crackers not only produces smoke of harmful gases but its harmful chemicals mixes with soil and pollute it, which makes the soil sterile and reduced growth of plants.
Question 9.
Write an essay on air pollution.
Answer:
Air pollution:
Any change in the composition of air is called pollution. The chief reason of air pollution is the releasing of harmful gas in the environment that affect our eyes, lungs, skins, heart and brain and produce various disorders in them.
Sources of air pollution : Following are the chief sources of air pollution:
1. Human sources:
- Combustion activities
- Industrial activities
- Agricultural works
- Use of solvents
- Activities concerned with atomic energy
2. Natural sources:
Volcano and its lava, ash, dust, smoke of forest fire, winds, cyclone. Decomposition of matters in the swamp water and liberation of methane gas and different compounds of hydrogen from forests plants, various pollen grains etc.
Effects of air pollution:
- Air pollutant like SO2 enter the soft tissues causing drying of the mouth, scratching throat and smarting eyes.
- Hydrocarbons and many other pollutants are responsible for causing cancer.
- Oxides of carbon, sulphur and nitrogen diffuse into the blood stream to combine with haemoglobin causing reduction in its oxygen carrying capacity. CO severely damages cardiovascular system and disturbs psychometry functions.
- RA.N. inhibits Hill reaction and thus, decreases photosynthetic production of an ecosystem.
Control of air pollution:
- Industrial smokes must be filtered before releasing it into the atmosphere.
- Tree plantation should be increased and deforestation prevented.
- Use of automobiles should be minimized which reduce the nitrogen contents in the atmosphere.
- The use of crude fuels should be avoided and use of high quality fuels should be recommended.
- Nuclear explosions should be avoided.
- Legal control of air pollution.
- Plantation of air purifying plants.
- Development of parks and gardens in cities.
Question 10.
Name the major air pollutants and their individual effects.
Answer:
Major air pollutants and their individual effects are as follows:
1. Carbon monoxide:
|It is highly poisonous gas. On entering blood stream, it combines with haemoglobin to block oxygen transport function. It causes laziness, headache, disturbance of psychometry functions, decrease in visual perception, serious effects on cardiovascular system etc.
2. Sulphur dioxide:
The animals and human population when exposed to SO2, suffer from respiratory diseases. It causes chest constriction, headache, vomiting and death from respiratory ailments.
3. Hydrogen sulphide:
Its rotten egg like smell cause nausea, irritation in eyes and throat.
4. Nitrogen oxide:
It inhibits cilia action so that soot and dust penetrate far into the lungs and finally resulting respiratory diseases in human beings and animals. In plants, it causing chlorosis.
5. Aerosoles:
These are the chemicals affecting ozone layer of the atmosphere due to which UV rays enter in the atmosphere and causing various diseases in plants and animals.
6. Ammonia:
It inflame upper respiratory passage in human beings. In plants, it inhibits seed germination, destruction of chloroplast and inhibition of the growth of roots and shoots.
7. Hydrogen chloride:
It affects the leaves of the plants, eyes, respiratory organs of animals and human beings.
8. Hydrocarbons:
It causes yellowing of plants, drying of buds, whereas in man and other animals, mucous glands of eyes and nose are irritated.
![]()
Question 11.
Write down the methods to control soil pollution.
Answer:
Methods to control soil pollution:
By following methods soil pollution can be prevented:
- Government should make provisions for latrines and people should not be allowed to litter in open fields.
- Solid wastes such as tin, copper, iron, glass etc., should not be dumped into soil.
- The solid wastes should be recycled to form new materials.
- Fertilizers and pesticides should be judiciously used.
- Instead of pesticides to kill pests, biological control methods should be adopted.
- The,excretory waste of man and cattle should be used to prepare biogas.
- Clean and well covered dustbins should be used in villages and cities.
- Closed hollow pipes should be used to collect and discharge liquid wastes.
- To prevent soil erosion, grass and small plants should be grown.
- Soil management should be adopted.
Question 12.
What are the sources of soil pollution?
Answer:
Sources of soil pollution:
- Acid rainwater and water from mines are main sources of soil pollution.
- Mixing of debris, waste products with the soil cause soil pollution.
- By excessive use of pesticides and fertilizers, pollute the soil.
- When industrial wastes are discharged in the soil, they also pollute the soil.
- Heavy metals example Cadmium, Zinc, Nickel, Arsenic etc., are mixed with the soil from mines. These metals are harmful for plants as well as primary and secondary consumers, in a food chain.
- Bones of dead animals, paper putrified flesh and food, iron, copper, mercury etc., pollute the soil.
- In our villages and rural areas, where there are no latrines, people go in fields for being at ease. From their stool also the soil is polluted.
- Insecticides like D.D.T. is very dangerous substance. When these enter the body of consumers from producers, their concentration is increased because these are non – degradable substances. Moreover, these can remain in the atmosphere for upto 15 years.
Question 13.
What is visible spectrum? Write short note on spectrum of solar light and also write effect of UV rays on the living organisms.
Answer:
The visible spectrum is the portion of the electromagnetic spectrum that is visible to the human eye. Electromagnetic radiation in this range of wavelength is called visible light or simply light. It ranges from 390 nm to 760 nm wavelength.
Different spectrum of sunlight (solar light):
Spectrum of sunlight can be divided into three parts :
- Ultraviolet spectrum – The UV spectrum covers the wavelength range 100 – 390 nm and is divided into three bands. It is invisible.
- Visible spectrum – It include light of ranges from 390 to 760 nm wavelength.
- Infrared spectrum – Light ranges 760 nm above is called as infrared ray. It is also invisible.
Effect of UV rays in the living organisms:
- It make DNA abnormal thus, protein sythesis in the body get effected.
- It may cause skin cancer.
- It supress immune system of the body.
- It causes sunburn and premature ageing of skin.
- It causes formation of harmful photochemical smog which causes respiratory diseases.
- It damage eyes.
Question 14.
Explain non – ionising and ionising radiation.
Answer:
Types of radiations on the basis of their action on cells, radiations are of two types :
1. Non – ionising radiations:
These include ultraviolet rays (UV rays; 100-300 nm), these have low penetration capacity. These affect only ‘those cells which absorb them. The cause are as following :
- Sunburn – It includes rupturing of sub – cutaneous blood capillaries, blisters, reddening of skin and injury to stratum germinativum.
- Snow blindness – It damages eyesight due to damage of corneal cells.
- Inactivation of organic bio-molecules and formation of thymin dimer in DNA.
- However they cause cancer, tumor, skin disease, etc.
2. Ionizing radiations :
These include X – rays, cosmic rays and atomic radiations.
These radiations have low wavelengths but high penetration power. These damage the living cells shifting the electron from one to other bio – molecule. Their harmful effects are as follows:
1. Short range effects : These may appear within few days or a few weeks after exposure. The effects include loss of hair, nails, subcutaneous bleeding, metabolic changes, change in proportion of blood cells, dead tissues or death of the organisms in high dose.
2. Long range effects : These appear in several months or even several years after their exposure. These cause tumours, cancers, mutations, genetic deformities, shorter life span and developmental defects.
Question 15.
What are sources of noise pollution? Write any four effects of noise pollution.
Answer:
Sources of noise pollution:
Noise is either natural such as thunder or man – made. The common sources of noise pollution are :
- Industries such as textile mills, printing-press, engineering establishments, etc.
- Defence equipment and vehicles such as tanks, artillery and rocket launching explosions, practise firing, etc.
- Transport vehicles as trains, trucks, buses, two – wheelers, jet planes, etc. and accessory noise produced by horns, sirens.
- Other applications as dynamite blasting, use of jack hammers, pile drivers, bulldozer, lawn mowers, etc.
Effects of noise pollution:
- The more acute and immediate effect of noise pollution is impairing of hearing leading to auditory fatigue and may finally lead to deafness.
- Interference with speech communication.
- Noise pollution leads to neurosis, anxiety, hypertension, cardiovascular disease, hepatic stress, giddiness.
- Annoyance leading to ill – temper, bickering, mental disorientation and violent behaviour.
- The high intensity of noise pollution can cause blood vessels to contract, skin becomes pale, muscles to contract and adrenaline to be shot into blood stream with consequence rise in blood pressure. This ultimately results in tension and nervousness.
- Affects different metabolic activities.
![]()
Question 16.
What is Greenhouse effect? Write any four impacts of Greenhouse effect
Answer:
Greenhouse effect:
The Greenhouse effect may therefore, be defined as “The progressive warming up of the earth’s surface due to blanketting effect of man – made CO2 in the atmosphere. It means the excessive presence of those gases blocked in the infrared radiation from earth’s surface to the atmosphere leading to an increase in temperature, which in turn would make life difficult on earth to forthcoming future generations.
Four impacts of Greenhouse effects are :
1. Effect on global climate:
The climate of earth has never been free of change. Its composition, temperature and self – cleansing ability have all varied since, the planet first formed. Yet the pace in the past two countries has been remarkable. The atmosphere’s composition in particular has changed significantly faster than it has at any time in human history.
Increase in Greenhouse gases does not increase the temperature of the earth i. e., it is not uniform. Temperature is normal at poles and is very less at tropics. In Iceland, Greenland, Sweden, Norway, Finland, Alaska, Siberia, temperature is more, thus at poles, ice starts melting.
2. Effect of Greenhouse Effect on forests:
When the atmospheric temperature increases, only those plants can survive which can bear high temperature. Because of this, new species of plant also come into existence, Herbaceous plant can not survive in this high temperature. Plants with high wood will increase in number. According to a survey if the amount of CO2 in the atmosphere is doubled, there will be a great decrease in a green biomass.
3. Effect on crops:
The positive aspect of greenhouse effect will be on agriculture. Because, CO2 is a natural fertilizer, the plants will grow larger and faster with increasing CO2 in the atmosphere. At first sight, the abnormal growth of plants might be expected to be beneficial because the yields of major crops might increase, however chances of depletion of characters and productivity of soil are also associated with this.
4. Effect on ozone layer:
During depletion, the chlorine, fluorine, or bromine molecules of CFCs and halogens are converted into reactive free radical form by photochemical reactions from their intial non – reactive sites. Oxides of nitrogen generally inactivate Cl but the lowering of stratospheric temperature changes NO2 into non – reactive nitric acid. Thus, Cl or F are free to react with ozone, distintegrating it into O2 + O. The tiny ice particles during winter favours the conversion of chlorine into chlorine monoxide, which behaves as a catalytic compound. The abundance of chloromonoxide rich air in stratosphere continues to rise. Chlorine monoxide react with nascent oxygen and thereby gets converted into chlorine. Thus, the cycle continues destroying the ozone level.