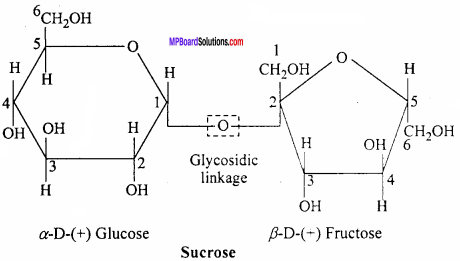
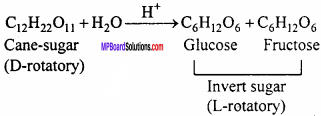
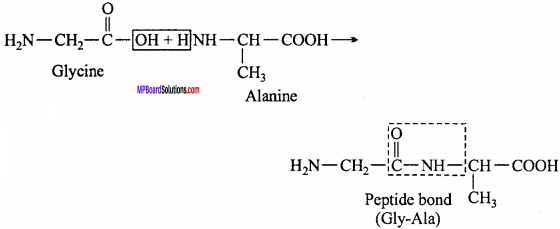
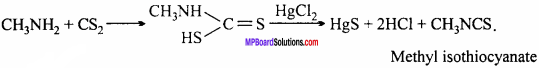
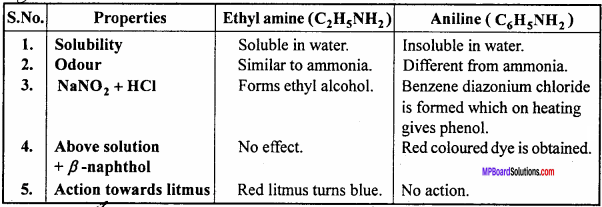
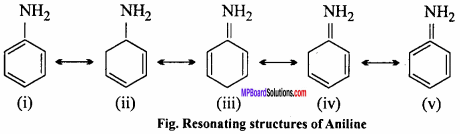
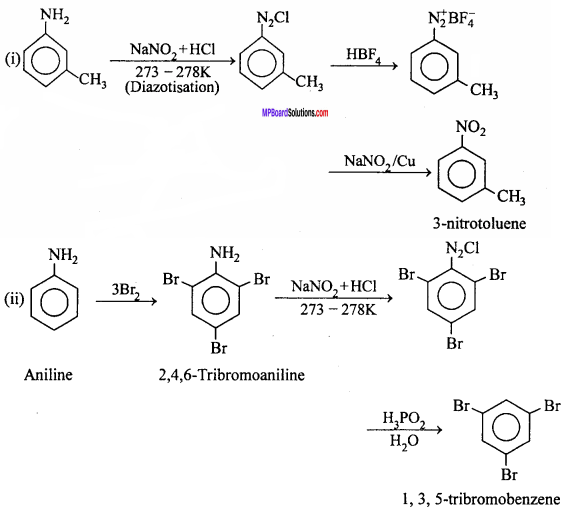
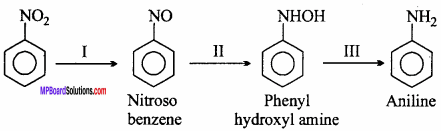
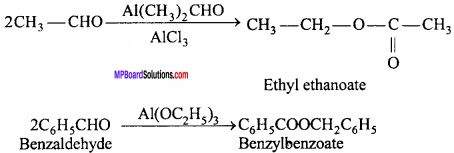
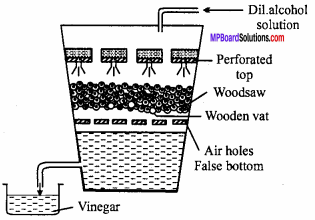
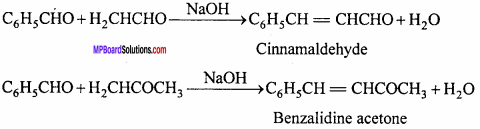
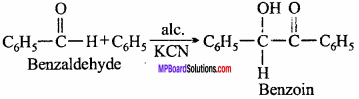
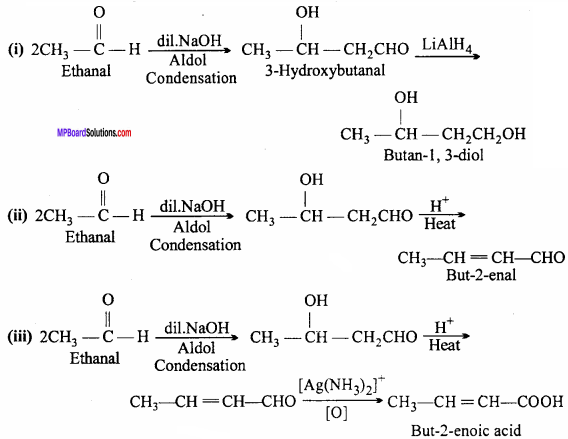
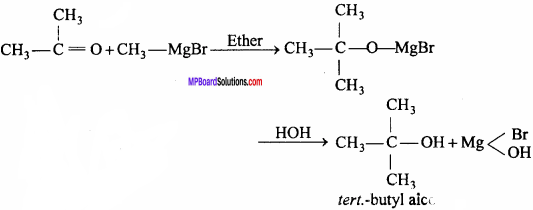
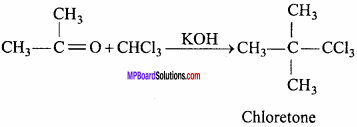
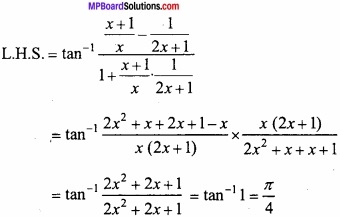
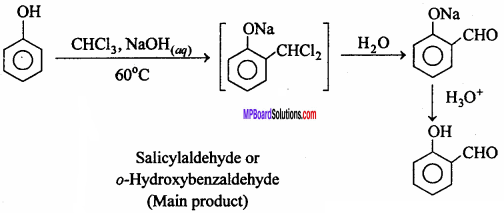
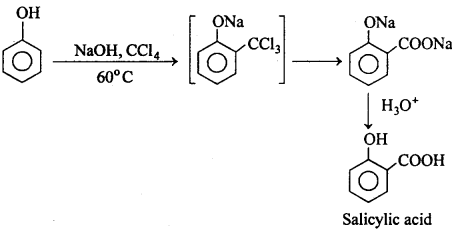
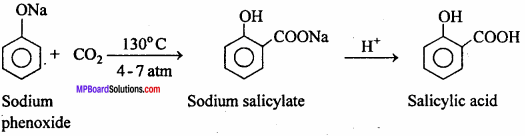
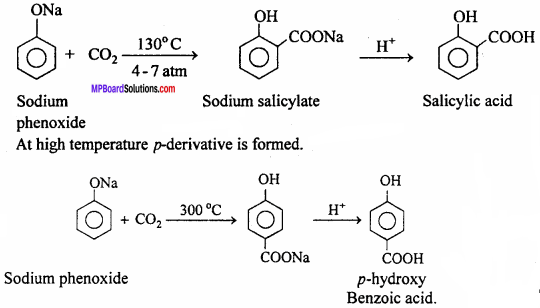
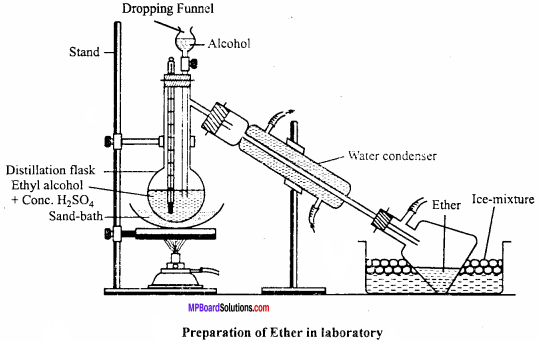
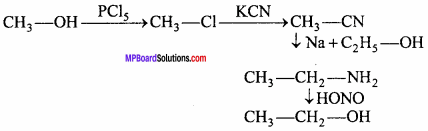
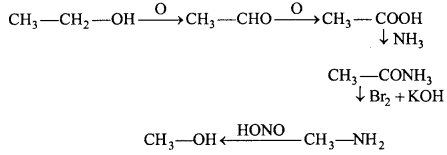
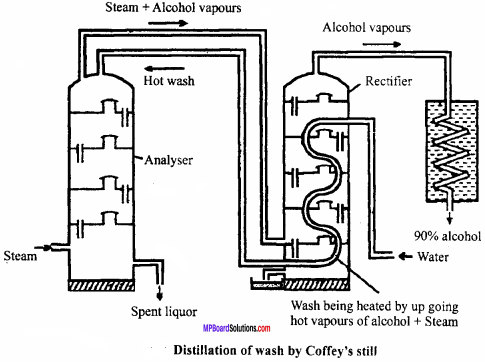
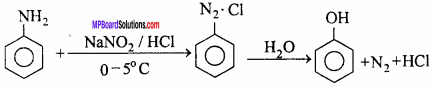
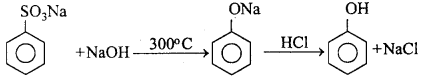
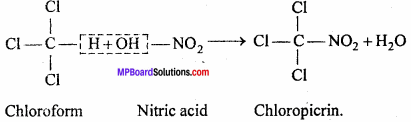
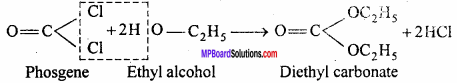
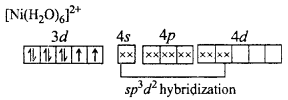
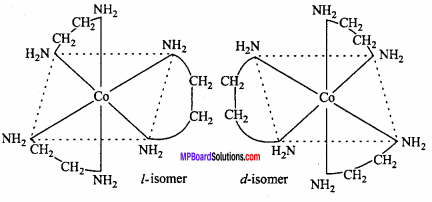
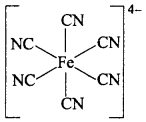
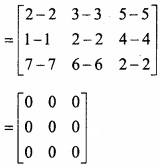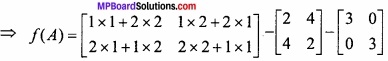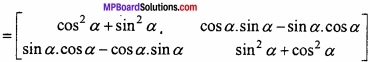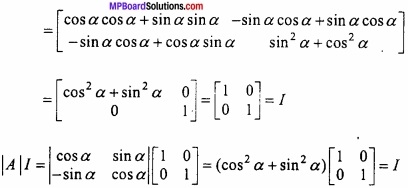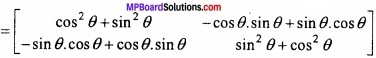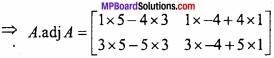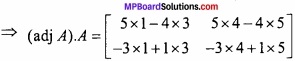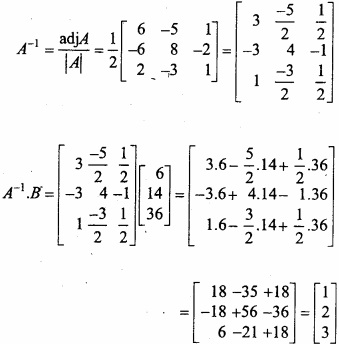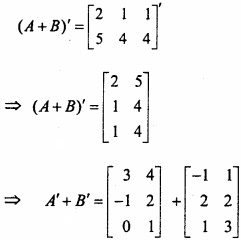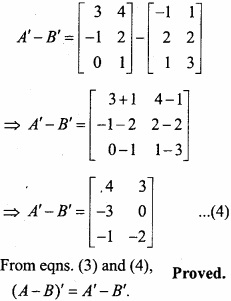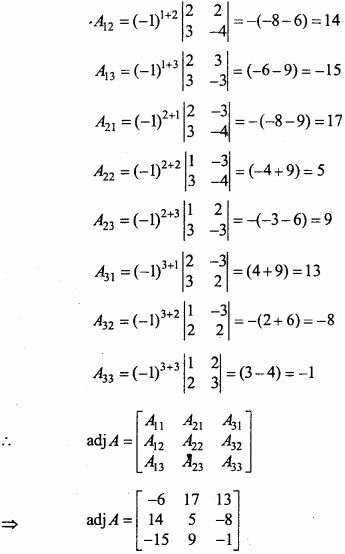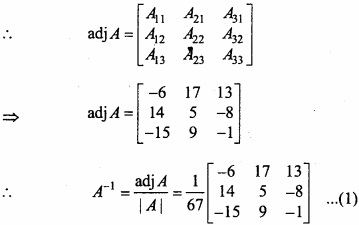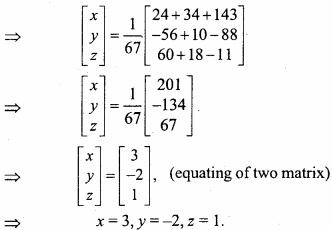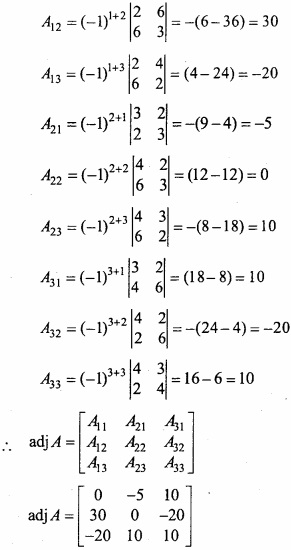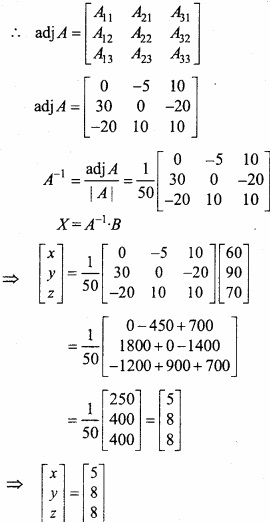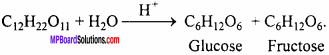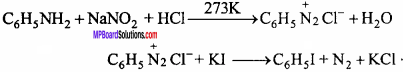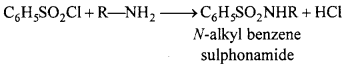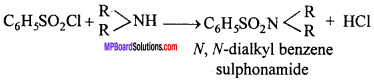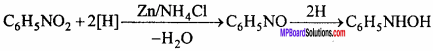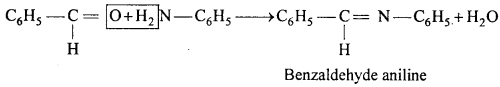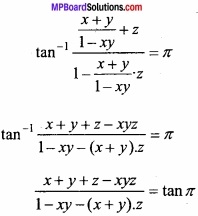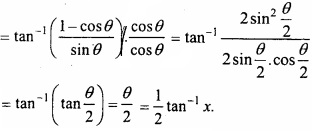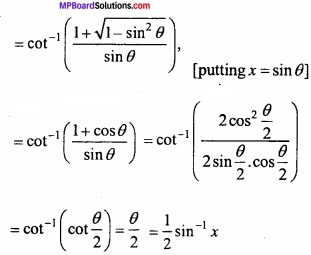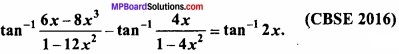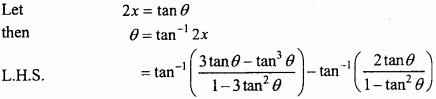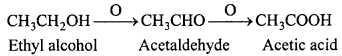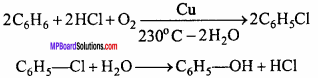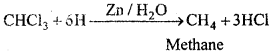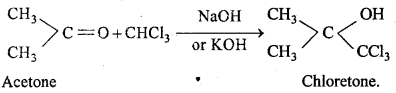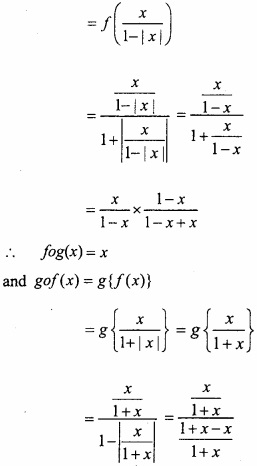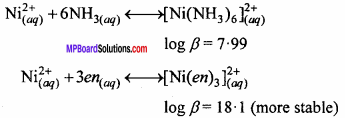MP Board Class 12th Chemistry Important Questions Chapter 15 Polymers
Polymers Important Questions
Polymers Objective Type Questions
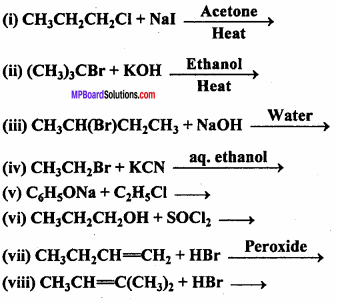
Question 1.
Choose the correct answer :
Question 1.
The polymerization in which two or more chemically different monomers take part is called :
(a) Addition polymerization
(b) Copolymerization
(c) Chain polymerization
(d) Homogeneous polymerization.
Answer:
(b) Copolymerization
Question 2.
Natural rubber is mainly a polymer of:
(a) Chloroprene
(b) Neoprene
(c) Isoprene
(d) Butadiene.
Answer:
(c) Isoprene
Question 3.
Which is a heat resistant polymer :
(a) P.V.C.
(b) P.V.A.
(c) Bakelite
(d) Rubber.
Answer:
(c) Bakelite
Question 4.
Is not a polymer :
(a) Orlon
(b) Teflon
(c) Neoprene soprene.
(d) Isoprene
Answer:
(d) Isoprene
Question 5.
Which among the following is a thermosetting polymer :
(a) P.V.C.
(b) P.V.A.
(c) Bakelite
(d) Perspex.
Answer:
(c) Bakelite
![]()
Question 6.
Which substance is used as ‘non stick’ in cooking utencils :
(a) P.V.C.
(b) Polystyrene
(c) Poly ethylene Pterephthalate
(d) Poly tetrafluoro ethylene.
Answer:
(d) Poly tetrafluoro ethylene.
Question 7.
Out of the following which polymer contain nitrogen :
(a) Nylon
(b) Polythene
(c) P.V.C.
(d) Terylene.
Answer:
(a) Nylon
Question 8.
Is a natural polymer :
(a) Starch
(b) Nylon
(c) Teflon
(d) Buna – S – Rubber
Answer:
(a) Starch
Question 9.
Is a polymer :
(a) Macro molecule
(b) Micromolecule
(c) Submicro molecule
(d) None of these
Answer:
(a) Macro molecule
Question 10.
P.V.C is a polymer of the following :
(a) CH2 = CH2
(b) CH2 = CHCl
(c) ClCH2 = CH2Cl
(d) Cl – C ≡ C – Cl
Answer:
(b) CH2 = CHCl
Question 11.
Teflon is a polymer of:
(a) Vinyl chloride
(b) Ethylene
(c) Acetylene
(d) Tetrafluroethene
Answer:
(d) Tetrafluroethene
Question 12.
Example of condensation polymeris :
(a) Polythene
(b) P.V.C.
(c) Orion
(d) Terylene
Answer:
(d) Terylene
![]()
Question 13.
Intermolecular force in elastomer is :
(a) Not present
(b) Weak
(c) Strong
(d) Extremely strong.
Answer:
(b) Weak
Question 14.
Complete hydrolysis of cellulose gives :
(a) D – Fructose
(b) D – Ribose
(c) D – Glucose
(d) L – Glucose
Answer:
(c) D – Glucose
Question 15.
Cellulose is a :
(a) Protein
(b) Fat
(c) Hormone
(d) Polysaccharide
Answer:
(d) Polysaccharide
Question 16.
Which of the following is a natural polymer :
(a) Starch
(b) Nylon
(c) Teflon
(d) Buna – s – Rubber
Answer:
(a) Starch
Question 17.
Nylon is an example of:
(a) Polyamide
(b) Polythene
(c) Polyester
(d) Polysaccharide
Answer:
(a) Polyamide
Question 18.
Nylon 6,6 is not a :
(a) Condensation polymer
(b) Co – Polymer
(c) Polyamide Bakelite is a polymer of:
(d) Homopolymer
Answer:
(d) Homopolymer
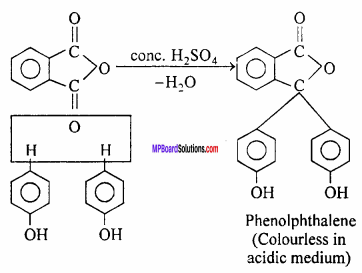
Question 19.
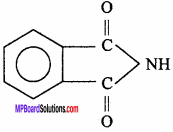
Bakelite is a polymer of :
(a) HCHO and acetic acid
(b) HCHO and phenol
(c) C2H5 – OH and phenol
(d) CH3 – COOH and benzene.
Answer:
(b) HCHO and phenol
Question 20.
Which of the following is a biodegradable polymer :
(a) Cellulose
(b) Polythene
(c) Polyvinyl chloride
(d) Nylon 6.
Answer:
(a) Cellulose
Question 2.
Fill in the blanks :
- ……………… is used for the preparation of chloroprene.
- Charge on polymers is ………………
- Polymer ……………… the light.
- Molecular mass of polymers is ………………
- Glucose is a monomer of ………………
- Cellulose is a ……………… polymer. (MP 2015)
- Polymer of ethylene glycol and phthalic acid is ………………
- Rubber is a ……………… polymer.
- Vulcanisation of rubber is an example of ………………
- Bakelite is a ……………… polymer.
- Nylon 6 is also called ……………… (MP 2011)
- Teflon is a polymer of ……………… (MP 2103)
Answer:
- Synthetic rubber
- Nil (zero)
- Scatter
- High
- Cellulose and starch
- Natural
- Glyptal
- Natural
- Elastomer
- Heat resistant
- Perlon – L
- Tetra fluoro ethylene.
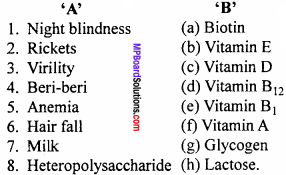
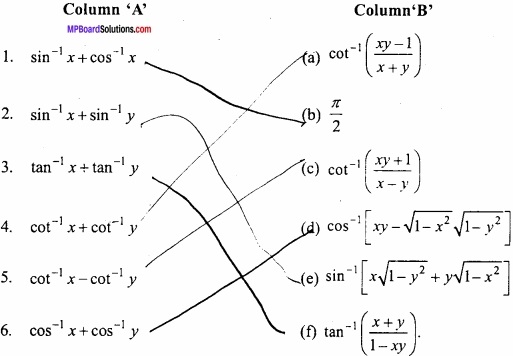
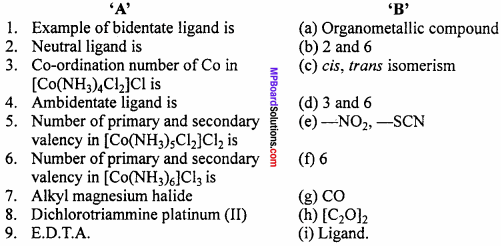
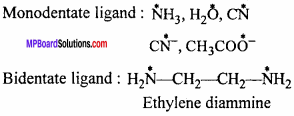
Question 3.
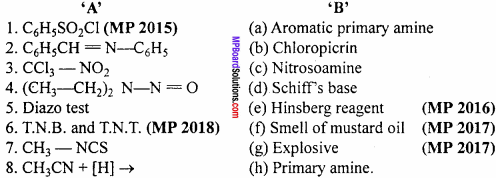
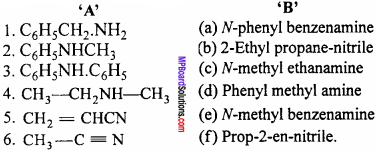
Make correct pairs :

Answer:
- (d)
- (a)
- (c)
- (f)
- (b)
- (g)
- (e)
- (h)
![]()
Question 4.
Answer in one word / sentence :
- Give two examples of natural polymer.
- Give two examples of addition polymer.
- Give two examples of condensation polymer.
- Write chemical name of Buna rubber.
- Give an example of synthetic rubber.
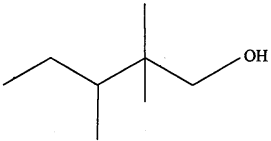
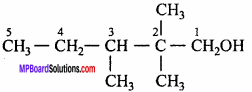
- Monomer of polythene is. (MP 2011)
- Name the polymer which is formed by condensation of ethylene glycol and dimethyl teraphthalic acid. (MP 2011)
- Name the polymerisation which takes place by addition of two or more than two different monomers. (MP 2010)
- Give the name of polymer used for formation of tyre thread. (MP 2010)
- Which polymer obtained by polymerisation of caprolactum?
Answer:
- Natural polymer – Rubber, starch
- Polythene, Polypropylene
- Nylon – 6, Bakelite
- Styrene Butadiene rubber
- Styrene Butadiene rubber (S.B.R.)
- Ethylene
- Terylene
- Copolymerisation
- Nylon – 6
- Nylon – 6
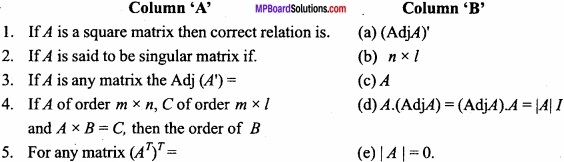
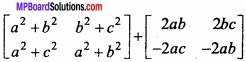
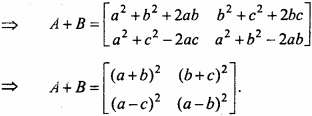

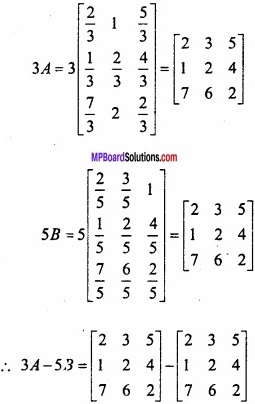
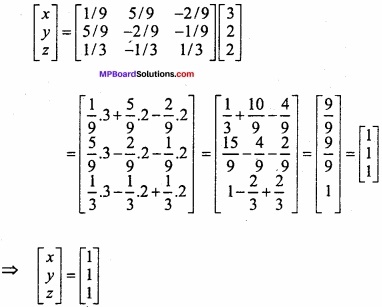
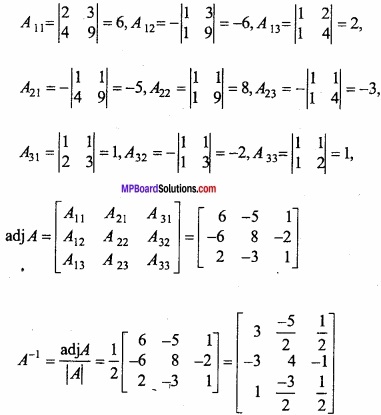
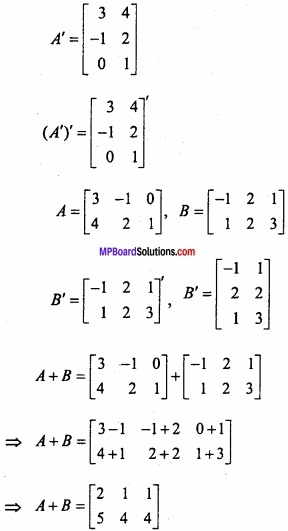
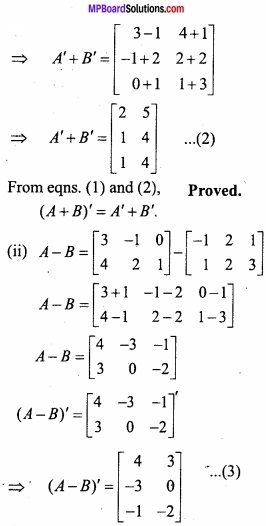
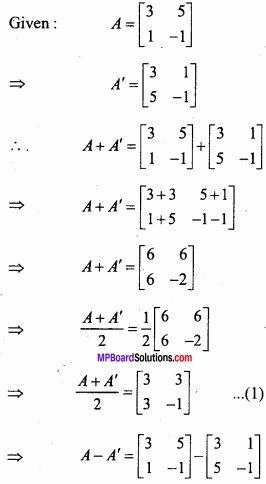
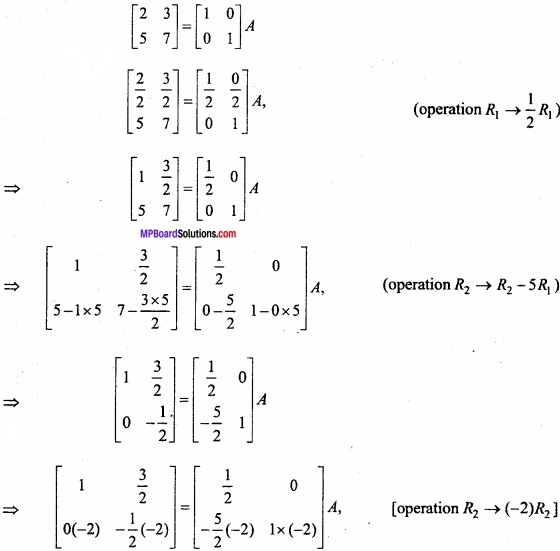
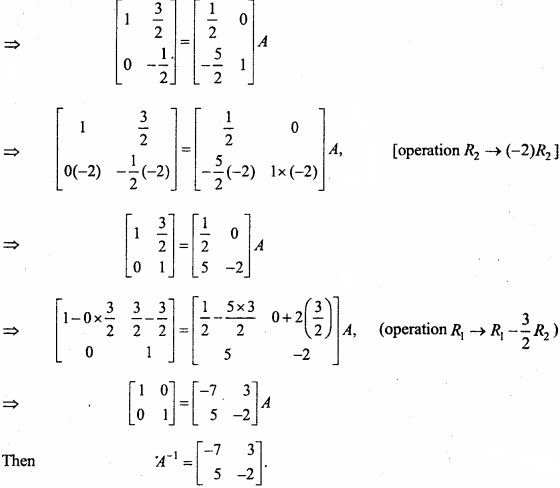
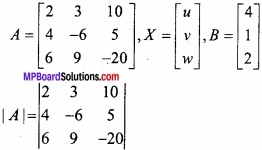
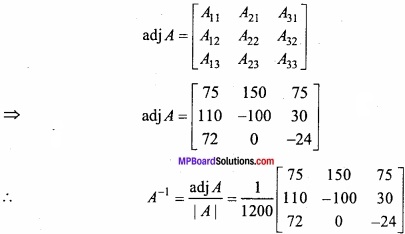
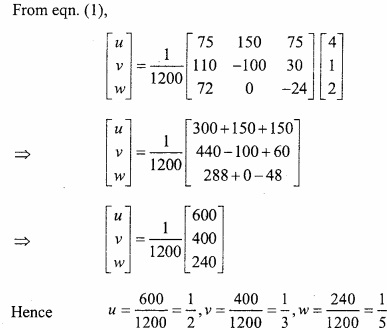
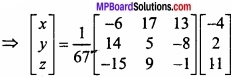
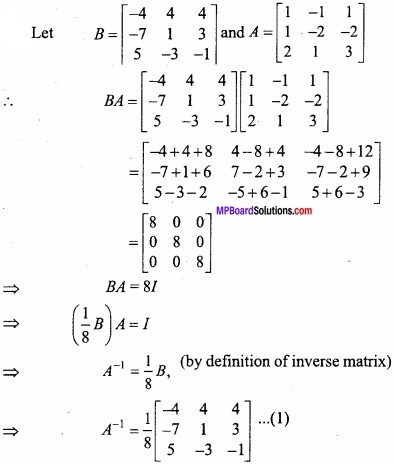
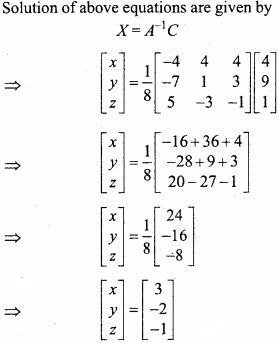
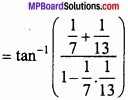
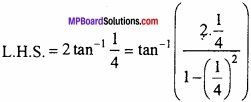
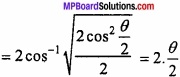
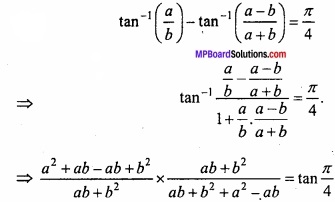
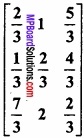 and B =
and B = 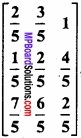 then find 3A – 5B? (NCERT)
then find 3A – 5B? (NCERT)