Students get through the MP Board Class 11th Chemistry Important Questions Chapter 12 Organic Chemistry: Some Basic Principles and Techniques which are most likely to be asked in the exam.
MP Board Class 11th Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
Organic Chemistry: Some Basic Principles and Techniques Class 11 Important Questions Very Short Answer Type
Question 1.
What are the hybridisation state of each carbon atom in the following com-pounds :
(i) CH2 = C = O,
(ii) CH3CH = CH2,
(iii) (CH3)2CO,
(iv) CH2 = CHCN,
(V) C6H6.
Answer:
(i) 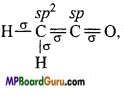
(ii) 
(iii) 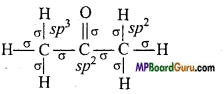
(iv) 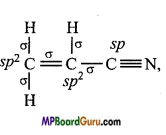
(v) 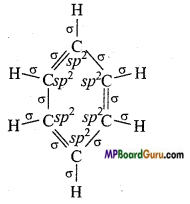
Question 2.
Identify the reagents shown in underline in the following equations as nu-cleophiles or electrophiles:
(a) CH3COOH + HO– → CH3COO–+H2O
(b) CH3COCH3 + CN– → (CH3)2C(CN)(OH)
(c) C6H5–+CH3CO → C6H5COCH3.
Answer:
(a) HO– is nucleophile,
(b) CN– is nucleophile,
(c) CH3 img is electrophile.
Question 3.
Classify the following reactions :
(a) CH3CH2Br + HS– → CH3CH2SH+ Br–
(b) (CH3)2C = CH2 +HCl → (CH3)2CCl-CH3
(c) CH3CH2Br + HO– → CH2 = CH2+H2O + Br–
(d) (CH3)3 C- CH2OH + HBr → (CH3)2CBrCH2CH3 +H2O.
Answer:
(a) Nucleophilic substitution reaction. Nucleophile (Br–) is substituted by other nucleophiles (HS–).
(b) Electrophilic addition reaction.
(c) β -Elimination reaction.
(d) Nucleophilic substitution reaction along with rearrangement.
Question 4.
What is Isomerism? Explain with example.
Answer:
Compound with same molecular formula but different structural formula are called isomers and this property is known as isomerism.
Example: Ethyl alcohol (C2H5OH) and dimethyl ether have same molecular formula C2H6O but their structural formulae are different, so they are isomers.
![]()
Question 5.
Explain position isomerism with example.
Answer:
Position isomerism: It includes those compounds in which there is a difference in the position of double or triple bond or in the linkage of the functional group with different carbon atoms.
Example: C3H8O:
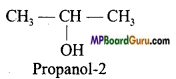
Question 6.
Write structural formulae of two functional isomers of molecular formula C3H6O.
Answer:
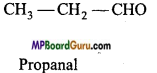
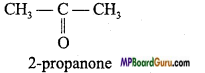
Question 7.
Write the isomers of pentane and write name of the isomerism present.
Answer:
C5H12 exhibits chain isomerism. It has three isomers :
1. CH3 – CH2 – CH2 – CH2 – CH3 w-pentane
2. 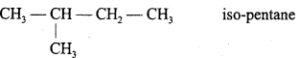
3. 
Question 8.
Explain chain and position isomerism in Alkyne.
Answer:
Alkynes represent chain and position isomerism.
1. Chain isomerism:
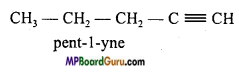
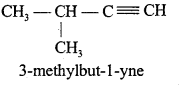
2. Position isomerism:
![]()
Question 9.
Which isomerism is found in Arenes? Give example.
Answer:
Arenes exhibit position isomerism.
Example: C8H10:
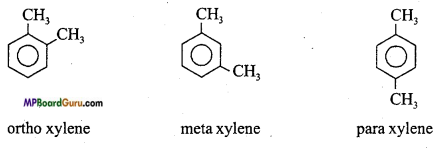
Question 10.
What type of isomerism exist in Alkane? Explain with example.
Answer:
Alkanes exhibit chain isomerism.
Examples : C5H12: CH3– CH2– CH2– CH2– CH3 n-pentane
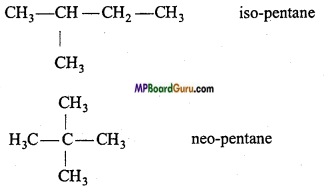
Question 11.
Give name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Answer:
Mixture of CaSO4 and camphor is separated by sublimation because camphor is sublime and CaSO4 is not. Thus, on heating camphor will deposit on the walls of the funnel and CaSO4 will remain in the crucible.
Question 12.
Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens.
Answer:
Nitrogen, sulphur and halogens present in organic compounds are in covalent state, therefore their identification is not easy. On fusing with Na metal, they get converted to NaCN, Na2S or NaX, that is they get converted to ionic form. Thus, in ionic form, these elements can be easily identified.
Question 13.
Explain, why an organic liquid vaporises at a temperature below its boiling point in its steam distillation?
Answer:
In steam distillation method, mixture of organic liquid and water boils when the sum of vapour pressure of the organic liquid (P1) and vapour pressure of water (P2) becomes equal to the atmospheric vapour pressure (P).
Thus, P = P1 + P2
Since, P1 is less than P, thus organic liquid starts boiling at a temperature below its boiling point.
![]()
Question 14.
Structural formula of the following compounds are given. Write the name of isomerism found in them.
1. CH3 – CH2 -CHO and 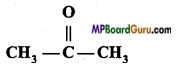
2. CH3– O- C3H7 and C2H5 – O -C2HS
3. ![]()
Answer:
1. Functional isomerism,
2. Metamerism,
3. Tautomerism.
Question 15.
What type of isomerism is shown by Alkenes? Explain with example.
Answer:
Alkenes show Chain, Position and Geometrical isomerism.
1. Chain isomerism: C4H8 :
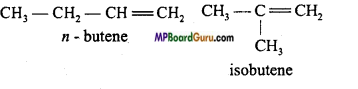
2. Position isomerism: C4H8
![]()
3. Geometrical isomerism : C4H8:
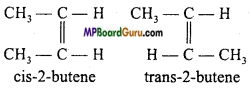
Question 16.
Explain ring chain isomerism with example.
Answer:
When molecular formula of two or more than two compounds is same but carbon in the compound may be arranged as an open chain or closed chain, then this type of isomerism are known as ring chain isomerism.
Example: C6H12:
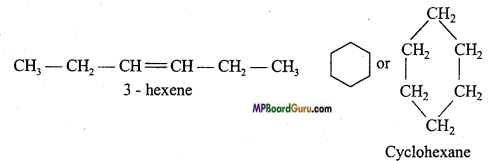
Question 17.
Write the method for the estimation of C and H in organic compounds.
Answer:
Liebig method is used for the estimation of C and H in organic compounds.
Question 18.
Write the name of method to purify the following mixtures :
- Impure Naphthalene,
- Two volatile liquids,
- Iodine and NaCI,
- Benzene and Toluene.
Answer:
- Impure Naphthalene: Sublimation.
- Two volatile liquids: Fractional distillation.
- Iodine and NaCl: Sublimation.
- Benzene and Toluene: Fractional distillation.
Question 19.
Write names of different methods used for purification and separation of organic compounds.
Answer:
For purification and separation of organic compounds different methods are following :
(A) For solid substances :
1. Crystallisation :
- Simple crystallisation,
- Fractional crystallisation.
2. Sublimation.
(B) For liquid substances :
- Simple distillation,
- Fractional distillation,
- Steam distillation,
- Vacuum distillation or reduced pressure distillation.
Question 20.
What is chromatography?
Answer:
The process in which different components of a mixture are separated by distributing in stationary or mobile phases on the basis of difference in adsorption abilities on any adsorbent is called chromatography.
Question 21.
If an organic compound is given in very small amount, then how is its purity identified?
Answer:
If an organic compound is given in very small amount, then its purity is identified by determining its melting or boiling point or it is done by column chromatography if one band is obtained, then the compound is pure, if more than one band is obtained, then compound is impure.
Question 22.
What is the use of adsorption coloured band process in the purification of organic compounds?
Answer:
Adsorption coloured band process is used in the separation of complex compounds like vitamins and hormones and in the identification of purity of substances.
![]()
Question 23.
Small quantity (1 or 2 drops) of cone. HNO3 is added when halogens are tested in presence of nitrogen. Why?
Answer:
When halogens are tested in presence of nitrogen, it creates problem. Therefore, one or two drops of cone. HNO3 is added previously which decomposes N and S. Both are liberated in the form of HCN and H2S gas.
NaCN + HNO3→NaNO3 +HCN ↑
Na2S + 2HNO3 → 2NaNO3 +H2S↑.
Question 24.
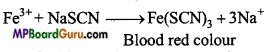
While testing for nitrogen in organic compounds sometimes blood red colour is obtained on adding FeCl3 solution. Why?
Answer:
If blood red colour is obtained while testing for nitrogen in the given organic compound, it means both N and S are present in the organic compound. If both N and S are present NaCNS is formed in the Na-extract which gives blood red colour with FeCl3.
Na + C + S + N → NaCNS
3NaCNS+FeCl3 →Fe(CNS)3 ↓ +3NaCl Ferric sulphocyanide (Blood red).
Question 25.
How can molecular mass of compounds be determined by volumetric analysis?
Answer:
By volumetric analysis, compounds of acidic or basic property is titrated in presence of an indicator by a standard base or acid, and their equivalent masses are determined. Standard acid or base is prepared by dissolving its equivalent mass in one litre.
Molecular mass of acid or base = Equivalent mass × Acidity or Basicity.
Question 26.
Why sodium extract is prepared for testing the elements of organic compounds? Give reason.
Answer:
The nature of organic compounds is covalent, as a result they do not ionise and thus it is difficult to test the presence of elements in it. Sodium is active metal which reacts with the elements present in the organic compound (N, S, Cl, Br, I) and forms sodium compounds which are ionic in nature and thus test of elements becomes easy.
Question 27.
Why is chloroform or CCl4 added in sodium extract for the identification of bromine or iodine?
Answer:
Sodium extract is neutralised by dilute HNO3 and CHCl3 or CCl4 and chlorine water is added. Chlorine displaces the bromine or iodine present in sodium extract which dissolves in CHCl3 or CCl4 and provides brown or violet colour.
Question 28.
How are carbon and hydrogen detected in an organic compound?
Answer:
In a test tube organic compound is heated with CuO, the CO2 gas evolved turns lime water milky whereas water vapour gets condensed in the form of drops, which give blue colour on being absorbed by CuSO4.
C + 2CuO → CO2 + 2Cu
2H + CuO → H2O + Cu
CuSO4 White + 5H2O → CUSO4 Blue .5H2O
CO2+Ca(OH)2 → CaCO3 Milky+ H2O
Question 29.
What do you underštand by mixed melting point process of an organic compound?
Answer:
Mixture of two completely, mixed substances is called mixed melting point. In this method melting point of mixture is determined. If it is same as the melting point of pure substance, the substance is pure.
Question 30.
How are two liquids with different boiling points separated?
Answer:
Mixture of two liquids which differ in their boiling points can be separated by fractional distillation. The more volatile liquid evaporates first passes through th condenser get condensed and collected in the receiver.
Vapours of less volatile liquid condense in the fractionating column and returns back. After the removal of more vohitile liquid at a particu lar temperature then the less volatile liquid gets distilled at a higher temperature.
Question 31.
What is empirical formula? Give its importance.
Answer:
Formula showing simplest whole number ratio of different constituents of compound is called empirical formula.
Example: Empirical formula of glucose is CH2O.
Question 32.
What Is molecular formula ? Explain with example.
Answer:
Chemical formula which expresses the actual number of atoms of various elements in a compound is called molecular formula.
Example: Molecular formula of glucose is C6 H12O6.
Molecular formula = Empincal formula × n, [ n = \(\frac{\text { Molecular mass }}{\text { Empirical formula mass }} \) ]
Question 33.
What are heterocyclic compounds? Explain with example.
Answer:
This series includes those cyclic compounds in which apart from carbon one or more polyvalent atoms are present like, Nitrogen, Sulphur, Oxygen etc
Example : (i) Pyridine, C5H5N
(ii) Furan C4H4O,
(iii) Thiophene C4H4S.
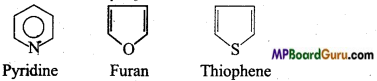
Question 34.
Will CCl4 give white precipitate of AgCI on heating with silver nitrate? Explain your answer with reason.
Answer:
CCl4 will not give white precipitate with AgNO3 solution because CCl4 is a covalent compound. It doés not ionise due to which Cl– ions are not available for the formation of precipitate of AgCl.
![]()
Question 35.
What are homocyclic compounds? Explain with example.
Answer:
This series include organic compounds which contain only carbon atoms in the cycle. Homocyclic compounds are of two types:
- Aromatic compounds
- Alicyclic compounds
Examples of Aromatic compound:
1. Benzene: C6H6 or![]()
2. Toluene: C6H5CH3 or 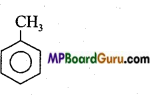
Examples of Alicyclic compound:
1. Cyclohexane: C6H12 or 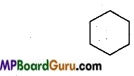
2. Cyclopentane : C5 H10 or 
Question 36.
What are functional groups?
Answer:
Every organic compound is made up of two parts. The part which determines the common properties or chemical properties of a particular homologous series is called a functional group. For example, -OH is the functional group present in alcohols C2H5OH.
Question 37.
Write general formula and name of first two members of Aldehyde series.
Answer:
General formula of Aldehyde series is R-CHO or CnH2n+1-CHO
Chemical formula Common name IUPAC name
HCHO Formaldehyde Methanal
CH3CHO Acetaldehyde Ethanal.
Question 38.
Write names and formulae of 5 carbon alkane, alkene and alkyne.
Answer:
1. Alkane – C5H12 – Pentane
2. Alkene – C5H10 – Pentene
3. Alkyne – C5H8 – Pentyne.
Question 39.
What is a radical?
Answer:
The part left after the removal of a hydrogen atom from a hydrocarbon is called radical. The part left after the removal of a hydrogen atom from alkane is called alkyl radical. It is represented by – R.
Example: CH3 – methyl, C2H5 – ethyl.
The part left after the removal of a hydrogen atom from an aromatic hydrocarbon is called aryl radical. It is represented by Ar.
Example: C6H5 – Phenyl.
Question 40.
Write name and formulae of first two members of Ester series.
Answer:
General formula of Ester series is RCOOR or CnH2O2
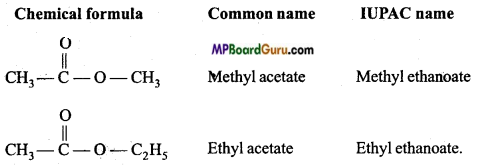
Question 41.
Give examples of Nucleophille and Electrophilic reagents.
Answer:
Nucleophilic reagents:
- Negative nucleophilic reagent: Cl–, Br–, SO4-2, OH–
- Neutral nucleophilic reagent: NH3, H2O, R – OH.
Electrophilic reagents:
- Positive electrophilic reagent NH4+, H3O+, Cl+ NO2+
- Neutral electrophilic reagent: BF3, AlCl3, FeCl3.
Organic Chemistry: Some Basic Principles and Techniques Class 11 Important Questions Short Answer Type
Question 1.
Why is it necessary to use acetic acid and not sulphuric acid for acidifica tion of sodium extract for testing sulphur by lead acetate test?
Answer:
If H2SO4 is used, then lead acetate itself reacts with H2SO4 to give white precipi
late of lead sulphate.
Pb(CH3COO)2 + H2SO4 → PbSO4 White ppt ↓+ 2CH3COOH .
Thus, white precipitate of PbSO4 will affect the following test of sulphur.
![]()
But, if acetic acid is used, then it does not react with lead acetate due to which no obstruction is produced in the test.
Question 2.
Why is a solution of Potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in organic compound?
Answer:
CO2 is slightly acidic in nature. Therefore, it reacts with strong base KOH to form K2CO3 by the help of which, percent amount of carbon in organic compound can be determined by the CO2 obtained.
2KOH + CO2 → K2CO3+H2O
By the increase in mass of U-tube of KOH, mass of CO2 can be determined. By the mass of CO2 obtained carbon present in organic compound can be determined as follows :
%C = \(\frac{12}{44}\) × \(\frac{\text { Mass of } \mathrm{CO}_{2} \text { produced }}{\text { Mass of organic compound }} \) × 100.
Question 3.
What are Nucleophiles?
Answer:
Nucleophiles or Nucleophilic reagents: Reagents which donate an electron pair in chemical reactions are called nucleophiles.
These are negative ions including carb-anion or neutral molecules containing ffee electron pairs.
Example: Negative nucleophiles: Cl–, Br–, I–, CN–, HO–, R-CH2.
Neutral nucleophiles: NH3, RNH2, H2O, ROH.
Nucleophilic reagents are generally represented by Nu.
Question 4.
What are electrophiles?
Answer:
Electrophiles or Electrophilic reagents: Reagents which can accept an electron pair in chemical reaction are called electrophiles.
These are positive ions including carbonium ion or neutral molecules in which electron deficient centres are present.
Example : Positive electrophiles : H+, Cl+, Br+, I+, NO2, R3C+, RN2.
Neutral electrophiles : SO3H, AlCl3, BF3.
Electrophilic reagents are generally represented by E+.
Question 5.
Write difference between Electrophilic and Nucleophilic reagents.
Answer:
Differences between Electrophilic and Nucleophilic reagent
| Electrophilic reagent | Nucleophilic reagent |
| 1. These are electron deficient. | Contain more electrons. |
| 2. Generally electrons are in valence shell. | Generally, 8 electrons are in valence shell. |
| 3. They are electropositive ions. | These are electronegative ions. |
| 4. Neutral molecules which have incomplete octet are electron pair acceptor. | They are electron pair donor. |
| 5. These are Lewis acids. | These are Lewis base. |
| 6. They attack the molecule where electron density is maximum. | They attack the place of minimum electron density. |
Question 6.
Explain Can’t Hoff and Le Bell rule of valency of carbon atom.
Answer:
Atomic number of carbon is 6. Its electronic configuration is 2,4. In this way its valence shell contains 4 electrons and its valency is 4.
According to Can’t Hoff and Le Bell, carbon is sp3 hybridised, therefore its structure is tetrahedral in which carbon atom is at the centre and the four valencies are situated at the four comers and bond angle between two valencies is 109° 28′.
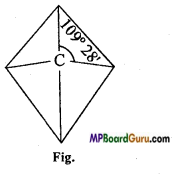
Question 7.
Carbon forms only covalent compounds. Why?
Answer:
Atomic number of carbon is 6 and its valence shell contains 4 electrons. It requires 4 extra electrons to complete its octet. Thus carbon can either donate 4 electrons or can accept 4 electrons or can share 4 electrons. But due to its small size its ionisation energy and electron affinity is very high.
Thus, it is not possible for carbon to donate or accept 4 electrons. Thus, to complete its octet carbon can only share electrons, therefore it forms only covalent compounds.
![]()
Question 8.
What is the reason of formation of large number of compounds by carbon? Or, Explain peculiar behaviour of carbon.
Answer:
Causes of existence of large number of carbon compounds are as follows :
1. Catenation: The tendency of atoms of an element to link with atoms of its own kind by covalent bond is called catenation. Tendency of catenation is maximum in carbon.
2. Strong bond between carbon-carbon atom: Size of carbon atom is very small. Due to it, overlapping between half filled orbitals of carbon is easier and more, as a result bond formed between carbon-carbon is very strong.
3. Tendency to form multiple bonds: Due to its small size, carbon can easily combine and form double and triple bond with other atoms. Like C, H, O, N.
4. Isomerism: When two or more than two compounds possess the same molecular formula but different structural formula, then such compounds are called isomers and the phenomenon is called isomerism. This property is maximum in carbon.
Due to these properties, carbon forms a large number of compounds.
Question 9.
Why is nitric acid added to sodium extract before adding silver nitrate for testing halogens?
Answer:
Sodium extract is boiled with concentrated nitric acid by which if NaCN and Na2S are present they dissociate.
NaCN + HNO3 → NaNO3 + HCN ↑
Na2S + 2HNO3→ 2NaNO3 + H2S ↑
If cyanide and sulphide are not removed, then they will react with AgN03 and obstruct the silver nitrate test of halogens.

Question 10.
Write the difference between Organic and Inorganic compound.
Answer:
Differences between Organic and Inorganic compound
| Organic compound | Inorganic compound |
| 1. These are formed by the combination of carbon and other elements like: H, O, N, S, P etc. | These compounds can be formed by all elements. |
| 2. These are covalent compounds. | Maximum inorganic compounds are electrovalent, but some compounds are covalent and also coordinate. |
| 3. These do not electrolyse. | These compounds can electrolyse. |
| 4. Their melting and boiling points are low. | Their melting and boiling points are high. |
| 5. These are insoluble in water but soluble in organic solvents. | These are soluble in water but insoluble in organic solvents. |
| 6. These exhibit isomerism. | These do not exhibit isomerism. |
| 7. Their reactions are molecular and complex and proceed slowly. | Their reactions are ionic and proceed fast. |
Question 11.
What is homologous series? Write its characteristics.
Answer:
Homologous series: A homologous series is a group of organic compounds with similar chemical constitutions and having a common functional group. Each member of series is called homologue. They have same chemical properties. The physical properties show a gradation.
Characteristics of homologous series :
- All the members of a particular series can be represented by a general molecular formula. For example, the alkanes or saturated hydrocarbons can be represented by a general formula CnH2n+2, where n is the number of carbon atoms.
- All the homologous of a particular series can be prepared by certain general methods.
- There is a common difference of one -CH2 group between any two consecutive members of series.
- All the members of a series have same chemical properties due to the presence of same functional group, e.g., all alcohols react with sodium metals liberating hydrogen gas.
- There is a regular gradation in the physical properties of the successive members of a particular series.
Question 12.
What are primary (1°), secondary (2°), tertiary (3°) and quaternary (4°) carbon atoms in the organic compounds?
Answer:
The classification of carbon atoms in alkane is done in the following manner:
- Primary carbon (1°) atom is that which is linked with one carbon atom.
- Secondary carbon (2°) atom is that which is linked with 2 carbon atoms.
- Tertiary carbon (3°) atom is that which is linked with 3 carbon atoms.
- Quaternary carbon (4°) atom is that which is linked with 4 carbon atoms.
In the following example primary (p), secondary (s), tertiary (t) and quaternary (q) carbon atoms are shown.

Question 13.
For the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
(a) CH3O – OCH3 → CH3O + ÒCH3
(b) >=O+–OH → > = O+H2O
(c) ![]()
(d) 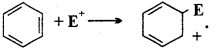
Answer:
(a) 
(b)
(c) 
(d) 
Question 14.
Explain the method of separation of a mixture of ammonium chloride and sodium chloride. Or, Explain the method of separation of a mixture of naphthalene and common salt.
Answer:
Mixture of ammonium chloride or naphthalene and NaCl is separated by sublimation. The mixture is placed in a porcelain dish covered with porous filter paper and a funnel is kept inverted over it. Mouth of the funnel is closed by cotton plugs. This is then heated slowly on a sand bath. NH4Cl or naphthalene sublimes pass through the pores of filter paper and get collected over the walls of the funnel from where it is collected and NaCl remains behind in the dish.
![]()
Question 15.
How nitrogen is tested in any organic compound by Lassaigne’s method?
Answer:
Test of nitrogen (Lassaigne’s test): In 2 ml of sodium extract, 2 ml of freshly prepared solution of FeSO4 is added along with little NaOH, warm it. Green precipitate of Fe(OH)2 is obtained. The solution is cooled and small quantity of cone. HC1 is added in which green ppt. dissolves. Then little amount of FeCl3 solution is added. If green or blue colour precipitate is obtained, then the substance contains nitrogen.
Na + C + N → NaCN
FeSO4 + 2NaOH → Na2SO4 + Fe(OH )2
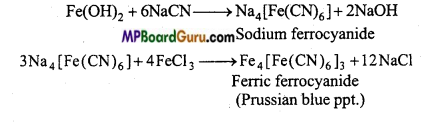
Question 16.
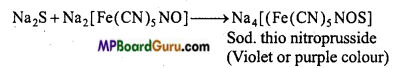
How is sulphur tested in an organic compound?
Answer:
Test of sulphur : (i) Sodium nitroprusside solution is added to the sodium extract of organic substance. If violet colour appears, it confirms the presence of sulphur in the given organic compound.
2Na+S → Na2S
(ii) If acetic acid and lead acetate solution is added to the sodium extract then a black precipitate is obtained, presence of sulphur is confirmed in it.
Na2S + (CH3COO)2 Pb → PbS ↓ Black + 2CH3COONa.
Question 17.
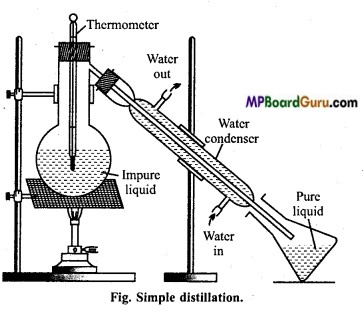
When is steam distillation more advantageous? Explain this method with diagram.
Answer:
Steam distillation: There are many organic compounds which are insoluble in water but are soluble in steam (water vapour). The distillation of such substances is called steam distillation. In this method impurities of non-volatile substance remain behind if the flask.
The impure mixture is taken in the flask and heated over sand bath and steam is passed through it. The steam along with vapour of substance passes through delivery tube and condenses. In receiver mixture of substance and water is collected. If organic substance is
solid, it is separated by filtration and if it is liquid then separation is done by separating funnel.
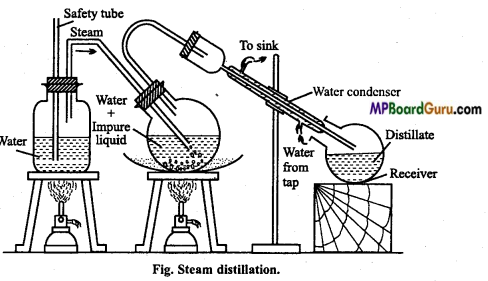
Question 18.
What is the relationship between the members of following pairs of struc tures? Are they structural or geometrical isomers or resonance contributors:
(a) ![]()
(b) 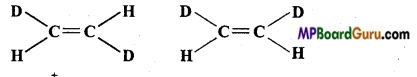
(c) 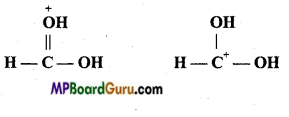
Answer:
(a) Structural isomers (Difference in position of functional group),
(b) Geometrical isomers.

(c) Resonance structures (position of electrons is different, but not of atoms.)
Question 19.
What is the principle of vacuum distillation or low pressure distillation? Explain with diagram.
Answer:
Vacuum distillation or Distillation under reduced pressure: Those organic compounds which decompose at their boiling point or even below the boiling point are purified by this method. As shown in the figure, partial vacuum is created in the receiver flask. This results in lowering of pressure in the distillation flask.
The liquid starts boiling at low temperature. Glycerol is purified by this method. At atmospheric pressure, its boiling point is 563 K but at a pressure of 12 mm, it boils at 453 K only.
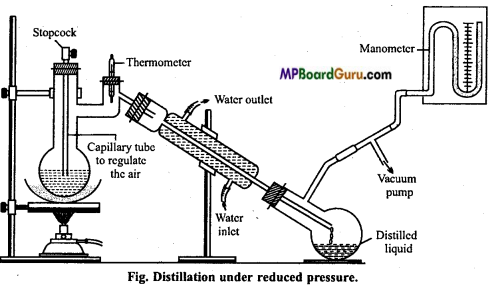
Question 20.
How are halogens present in organic compounds detected by silver nitrate test? Explain giving equation.
Answer:
Test of halogens by Silver nitrate test: To a part of sodium extract add cone, nitric acid and the silver nitrate solution. A curdy white precipitate shows the presence of chlorine, yellowish-white and lemon yellowish precipitates show the presence of bromine and iodine respectively.
NaCl + AgNO3 → NaNO3 + AgCl ↓ (curdy white precipitate)
NaBr + AgNO3 → NaNO3 + AgBr ↓ (yellowish white precipitate)
Nal + AgNO3→ NaNO3 + Agl↓ (lemon yellowish precipitate)
White precipitate of AgCl dissolves easily in dilute NH4OH to form a soluble complex.
AgCl + 2NH4OH → [Ag(NH3)2]Cl + 2H2O
Yellowish white precipitate of AgBr is sparingly soluble in NH4OH, hence dissolves with difficulty to form a soluble complex.
AgBr + 2NH4OH → [Ag(NH3)2 ]Br + 2H2O
Lemon yellowish precipitate of Agl is insoluble in NH4OH as well as in cone. HNO3.
Question 21.
Which of the following represents the correct IUPAC name for the compounds concerned:
(a) 2,2-Dimethylpentane or 2-Dimethylpentane.
(b) 2,4,7-Trimethyloctane or 2,5, 7-TVimethyloctane.
(c) 2-Chioro-4-MethyIpentane or 4-Chloro-2-MethyIpentane.
(d) But-3-yn-l-ol or But-4-oI-l-yn.
Answer:
(a) 2, 2-Dimethylpentane (Because for two alkyl groups on the same carbon atom, numbering should be twice).
(b) 2,4,7-Trimethyloctane (Because value of 2,4,7 numeral set is lesser than 2,5,7 numeral set.)
(c) 2-Chloro-4-Methylpentane (Because substituted groups are written in alphabetical order).
(d) But -3-yn-l-ol (Because functional group or alcohol group possess the lowest number).
Question 22.
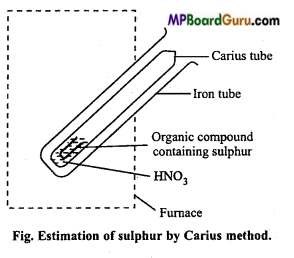
How is sulphur present in organic compounds estimated?
Answer:
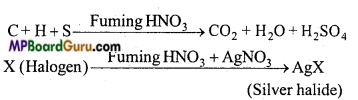
Estimation of sulphur: Sulphur is also estimated by Carius method. In this case, organic substance is heated with only HNO3 (AgNO3 is not used). Carbon, hydrogen and sulphur present in the organic compound gets oxidized to CO2, H2O and H2SO4 respectively on account of oxidation.
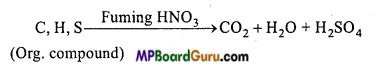
Carius tube is cut and contents removed by washing. Solution is filtered and washed well. Solution is warmed and treated with excess of BaCl2 solution, when a white precipitate of BaSO4 is obtained. Heat the ppt. at low temperature for sometime for aglomerization. Precipitate of BaSO4 is filtered, washed, dried and weighed. Percentage of sulphur is then calculated.
H2SO4 + BaCl2 → BaSO4↓ White precipitate + 2HCL
% of sulphar = \(\frac{32}{233} \) × \(\frac{\text { Weight of } \mathrm{BaSO}_{4}}{\text { Weight of org. compound }}\) × 100
Question 23.
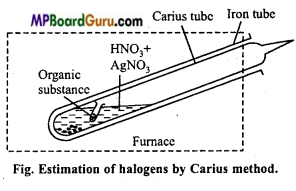
How are halogens present in organic compound estimated?
Answer:
Estimation of halogens: Canus method: In this method estimation of halogens (Cl, Br and I) is done. A known weight of organic compound containing halogen is heated with AgNO3 and fuming nitric acid. Carbon, hydrogen and sulphur present in the compound gets oxidized and halogens form AgX (Silver halide).
Observation: Mass of Silver halide = Mgm
Mass of organic compound = Wgm
% amount of Cl = \(\frac{35 \cdot 5}{143 \cdot 5}\) × \(\frac{\text { Mass of } \mathrm{AgCl}}{\text { Mass of organic compound }} \) × 100
%amount of Br = \(\frac{80}{188}\) × \(\frac{\text { Mass of } \mathrm{AgBr}}{\text { Mass of organic compound }}\) × 100
% amount of l =\(\frac{127}{235}\) × \(\frac{\text { Mass of AgI }}{\text { Mass of organic compound }}\) × 100
![]()
Question 24.
Write the principle of Kjeldahl’s method.
Answer:
Principle: Nitrogen-containing organic compound is heated with concentrated sulphuric acid in presence of K2SO4 and CuSO4. The nitrogen present is converted into ammonium sulphate. On heating ammonium sulphate with NaOH, the whole of N2 is changed into ammonia gas.
The NH3gas is passed into a known volume of standard solution of sulphuric acid. NH3 neutralises sulphuric acid. The amount of unreacted sulphuric acid is determined by titrating it with a standard alkali. From this percentage of N2 is calculated.
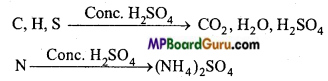
(NH4)2 SO4 +2NaOH → Na2SO4 +2NH3 + 2H2O
2NH3+H2SO4 → (NH4)2SO4.
Question 25.
Explain distillation method of purification of compounds.
Answer:
The various types of distillation are :
- Simple distillation,
- Fractional distillation,
- Vacuum distillation,
- Steam distillation.
Simple distillation: This method is employed for the purification of those liquids which, boil without decomposition and are associated with non-volatile impurities.
Liquids which have a difference of 30-40°C in their boiling points are purified by this method. On heating the mixture, vapours of pure substance are formed which condenses as they pass through the air or water condenser.
The pure liquid collects in the receiver while the non-volatile impurities are left behind in the flask. Some glass beads are also added to the distillation flask to avoid bumping.
Question 26.
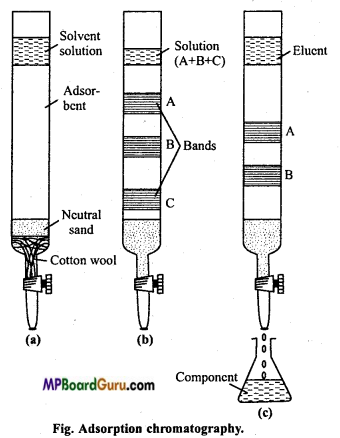
What is the principle of adsorption chromatography? Explain with diagram.
Answer:
Adsorption chromatography: It is also known as column chromatography. It is based on the fact that when solution of mixture comes in contact with some adsorbent, different components of a mixture get adsorbed to different extent on account of difference in power of adsorption.
The mixture to be separated and purified is first dissolved in a suitable non-polar organic solvent like petroleum ether, benzene, chloroform, alcohol, etc. This solution is allowed to flow down an absorption column.
For using this technique there should be proper adsorption column. Adsorbents like activated magnesia, alumina, calcium carbonate, gypsum, etc. is filled in a hard vertical tube (adsorbent column.).
Question 27.
Explain chain isomerism and functional isomerism with example.
Answer:
1. Chain isomerism: When the same molecular formula represents two or more compounds which differ in the nature of carbon chain (carbon skeleton) then such compounds are called chain isomers and the phenomenon is called chain isomerism.
Example : (a) C4H10 (butane) has two chain isomers.
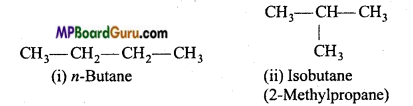
(b) C5H12 (pentane) has three chain isomers.
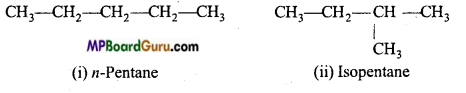
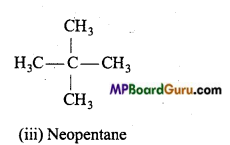
2. Functional group isomerism: When the molecular formula represents two or more compounds which differ in the nature of the functional group, then such compounds are called the functional isomers and the phenomenon is called functional group isomerism. Example :
(a) C3H8O : CH3 – CH2 – CH2 – OH
(i) Propanol
CH3 -O – C2H5
(ii) Ethyl methyl ether
(b) C4H8O:
![]()

Question 28.
Write a note on carbocation and carbanion.
Answer:
Carbocations or Carbonium ions: Carbocations are positively charged carbon atoms having only six electrons in its valence shell. They are also highly reactive and act as reaction intermediate. They are also called carbonium ions. They are produced by heterolytic cleavage. Some examples are :

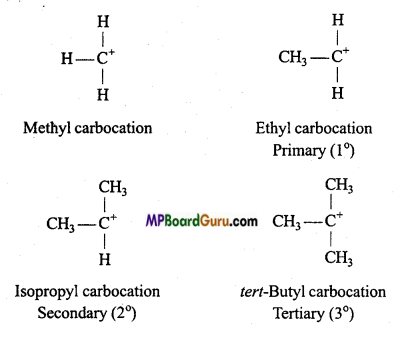
Types of carbocations: Carbocations are classified as primary, secondary or tertiary depending upon the nature of carbon atom carrying the positive charge.
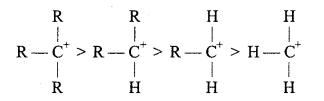
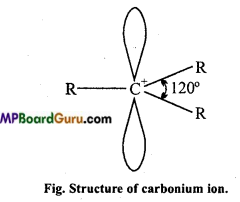
Stability of Carbonium ion: Larger the dispersal of positive ion on other groups or atoms, larger is the stability of carbonium ion. With the increase in the number of alkyl group its stability increases.
Structure: Positively charged carbon atom is sp2 hybridised and one p-orbital is vacant.
![]()
Question 29.
Give condensed and bond line structural formulae and identify the functional groups present if any, for :
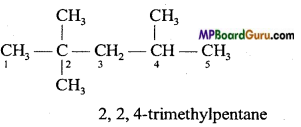
(a) 2, 2,4-Trimethylpentane.
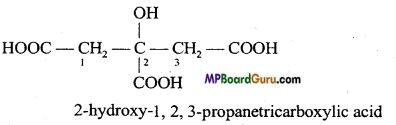
(b) 2-Hydroxy-1, 2,3 propane tricarboxylic acid.
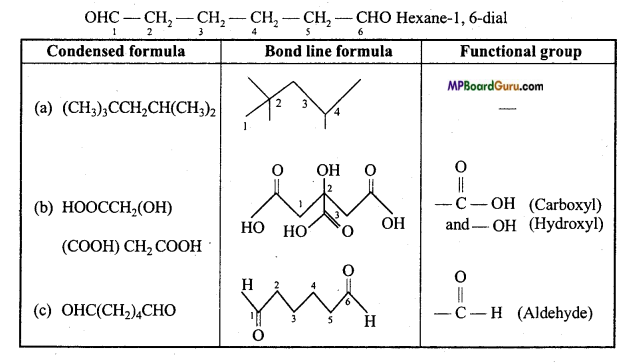
(c) Hexanedial.
Answer:
To write the condensed and bond line formulae first let us write the structural formulae of the compounds :
(a)
(b)
(c)
Question 30.
Write a note on carbanion or cabon anion.
Answer:
Carbanions: Carbanions are negatively charged carbon surrounded by eight electrons in the valence shell. They are also short-lived and highly reactive. They also act as reaction intermediates. They are also called carbon anions.
Methyl carbanion: H3C– :
Ethyl carbanion :
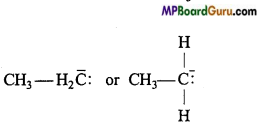
Types of carbanions: Carbanions are classified as primary, secondary and tertiary depending upon the nature of carbon carrying negative charge.
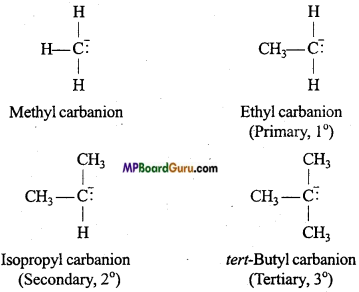
Relative stability of carbanion: Carbanions are electron rich species and hence their stability depend on dispersal of electrons away from the carbon carrying negative charge. The order of stability of alkyl carbanion is as follows :
Methyl carbanion > Primary carbanion > Secondary carbanion > Tertiary carbanion
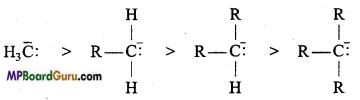
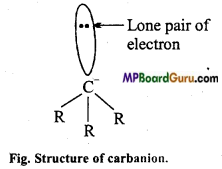
Structure: In it, carbon is in sp3 hybridised state, three bond paired electrons and one lone pair electron is present.
Lone pair of electron
Question 31.
Explain Metamerism and Tautomerism with example.
Answer:
Metamerism: When the same molecular formula represents two or more compounds which only differ in the nature of the alkyl groups attached to the same functional group, then such compounds are called metamers and the phenomenon is called metamerism.
Example: Molecular formula, C4H10O (ether) has three metamers :
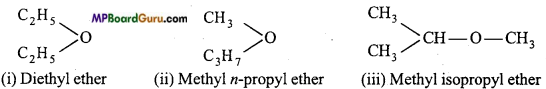
Tautomerism: This is a special type of functional isomerism in which one compound
exhibits two isomers. Both of the isomers are present in equilibrium. It is a dynamic state in which both isomers change into each other.
These are called tautomers and the phenomenon is known as tautomerism.
Example : (i) Diad system: Hydrogen vibrates between two multivalent atoms.
H – C ≡ N
![]()
(ii) Triad system: Hydrogen vibrates between three multivalent atoms.

Question 32.
What Is Fractional crystallisation?
Answer:
When two or more constituents of an impure mixture differ in the amount of their solubility in the same solvent, they are separated by fractional crystallisation process. Let the two constituents of the mixture are A and B but solubility of A is less than B. Saturated solution of this mixture is prepared in a suitable solvent like alcohol any filtered. The solution is then cooled.
A being less soluble forms crystals first which are separated by filtration. Then the solution is cooled. Crystals of B are obtained which are separated by filtration. This process is repeated several times because crystals obtained in the first time still contain impurities.
![]()
Question 33.
What is inductive effect? What is its use?
Answer:
Inductive effect: In a covalent bond between two dissimilar atoms having different electronegativities, the electron pair does not remain in the centre but get attracted towards the more electronegative atoms. The bond becomes somewhat polar due to unequal
sharing of the electron pair. For example, in the bond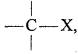 if X is more electronegative than C, the electron pair get attracted towards X. This shifting of electrons develops a partial negative charge denoted by δ– on X and C attains a partial positive charge denoted by δ+. Thus,
if X is more electronegative than C, the electron pair get attracted towards X. This shifting of electrons develops a partial negative charge denoted by δ– on X and C attains a partial positive charge denoted by δ+. Thus,
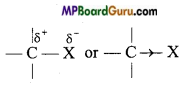
Now consider a long chain of carbon atoms with a more electronegative element say chlorine attached at one end.
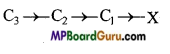
The electron pair of the bond between C1 and X gets displaced towards electronegative chlorine atom. This results in developing of partial negative charge on chlorine and partial positive charge on carbon. This displacement is further transmitted to other carbon atoms of the chain but the magnitude of displacement goes on decreasing with the increase in the distance of the carbon atoms from the chlorine atom as shown below :
![]()
(Here δδδ+ < δδ+ < δ+ )
Thus, it can be concluded that a polar bond induces polarity in the other covalent bonds in a chain. This type of displacement of electrons is referred to as inductive effect (or I effect) or transmission effect. Thus, inductive effect may be defined as, the permanent displacement of electrons along the chain of carbon atoms due to the presence of a polar covalent bond in the chain.
Types of inductive effect: There are two types of inductive effect:
(a) Electron withdrawing inductive effect (-1 effect): If the substance attached to the end of the carbon chain is electron-withdrawing, the effect is called -I effect. The de¬creasing order of-I effect of some atoms or groups is as follows :
– NO2 > -C ≡ N> -F> -COOH> -Cl> -Br>-I> -OCH3> – C6H5 > – H
(b) Electron releasing inductive effect (+1 effect): If the substance attached to the end of the carbon chain is electron releasing, the effect is called +1 effect. Alkyl groups are electron releasing in nature. Thus, the decreasing order of+1 effect is as follows :

Uses :
- Comparison of strength of fatty acids
- Basic character of amines
- Presence of dipole moment.
Question 34.
Write IUPAC name of the following compounds :
(a) 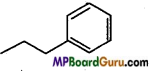
(b) 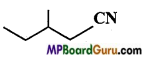
(c) 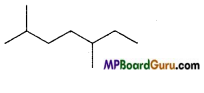
(d) 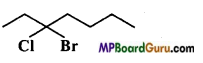
(e) 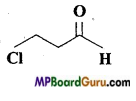
(f) Cl2CHCH2OH
Answer:
(a) 
(b)
(c) 
(d) 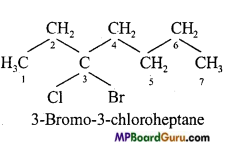
(e) 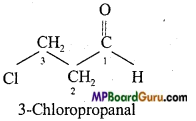
(f) 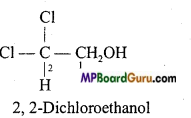
Question 35.
What do you understand by Electromeric effect? Write a note on it.
Answer:
Electromeric effect: It is a temporary effect which takes place in compounds containing multiple covalent bonds (e.g., C = C, C = O, C = N, etc.) or an atom with a lone pair of electrons adjacent to a covalent bond.
It is known to us that a multiple bond consist of σ – and π – bonds and the π -bonding electrons in π -bonds are loosely held.
Therefore, when a charged species approaches compound having double or triple bond, the electrons of the π – bond due to electrostatic attraction are completely polarized or displaced towards one of the constituent atoms. This transference of electron pair is shown by a curved arrow, starting at the original position of the electron pair and ending to the new position of the electron pair as shown below :

Types of Electro meric effect: There are two types of electromeric effects :
- +E effect: When the transfer of electron takes place towards the atom of the double bond to which the attacking species gets finally attached, the effect is called +E effect. Like -F, -NR2, -OR etc.
- -E effect: When the transfer of electron takes place away from the atom of the double bond to which the attacking species finally gets attached, the effect is called -Eeffect.
![]()
Uses:
- Addition reaction of halogen on unsaturated hydrocarbons (Alkene and Alkyne)
- Reaction of halogen acid on alkene and alkyne containing carbonyl group.
Question 36.
Write the difference between Inductive effect and Electromeric effect.
Answer:
Differences between Inductive and Electromeric Effect
| Inductive effect | Electromeric effect |
| 1. It is a permanent effect. | It is a temporary effect. |
| 2. This effect is produced due to displacement of electrons of σ -bond. | This effect is produced due to displacement of π -bonded electrons. |
| 3. Partial positive charge and partial negative charge is produced. | Complete positive and negative charge are produced. |
| 4. It leads to substitution reactions. | It leads to addition reactions. |
| 5. It always exists in the molecule. | This effect occurs in the presence of attacking reagent during the reaction. |
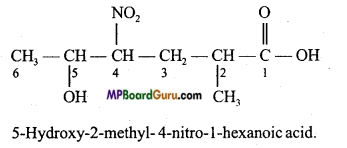
Question 37.
Explain the rules of nomenclature of compounds containing functional group with example.
Answer:
Rules of Nomenclature:
- The carbon chain containing the functional group is considered to be the main chain.
- If functional group contains carbon then it is counted in the main chain.
- Numbering of chain is started from the side which the functional group is nearer.
- During naming the substituents are named first and then the main functional group.
- If more than one functional group is present in the chain then on the basis of priority the functional group is selected.
- Then before naming the substituents, leaving the main functional group, position of other functional group and their names are written.
Question 38.
Represent σ – and π -bond in the following :
(i) C6H6,
(ii) C6H12,
(iii) CH2C12,
(iv) CH2 =C = CH2,
(V) CH3 NO2,
(vi) HCO – NHCH3
Answer:
(i)
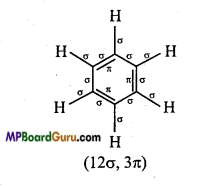
(ii) 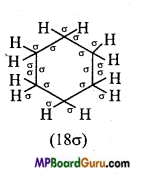
(iii) 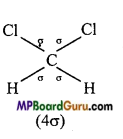
(iv) 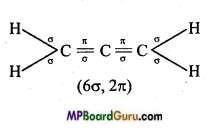
(v) 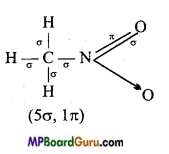
(vi) 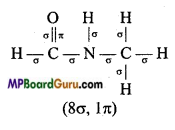
Question 39.
Write IUPAC name and formula of the following compounds :
1. Iodoform
2. Vinegar
3. Formic acid
4. Marsh gas
5. Acetone
6. Glycol
Answer:
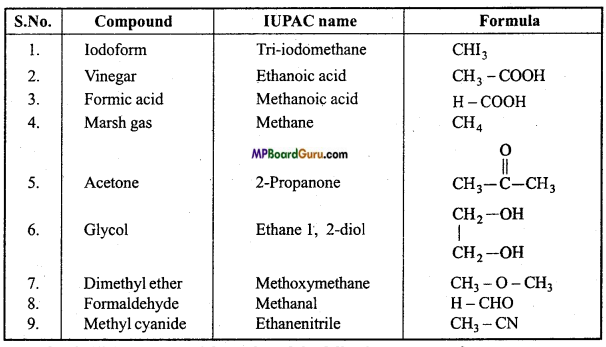
Question 40.
Write structural formulae of the following compounds :
1. 3, 3-Dimethylpentene,
2. 4-Methylhexan-2-ol,
3. 1, 2-Dibromoethane,
4. Nitromethane,
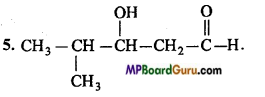
5. 4-Methyl-3-hydroxypentanal.
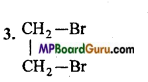
Answer:
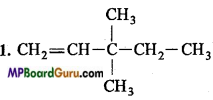
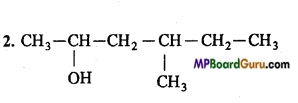
4. CH3 – NO2
Question 41.
Write the difference between Aliphatic compound and Aromatic compound.
Answer:
Differences between Aliphatic compound and Aromatic compound
| Aliphatic compound | Aromatic compound |
| 1. These are open-chain compounds. | These are closed chain compounds. |
| 2. These normally possess C- C bond. | These possess alternate single and double bond in the ring. |
| 3. These are more reactive. | These are less reactive. |
| 4. These do not undergo halogenation, nitration, sulphonation easily. | These undergo halogenation, nitration, sulphonation easily. |
| 5. Their heat of combustion is more. | Their heat of combustion is less. |
| 6. Their -OH radical are neutral. | Their hydroxy compounds represent acidic property. |
| 7. Their radicals are basic. | Their radicals are acidic. |
Question 42.
What is resonance effect? Write its application.
Answer:
Resonance effect is defined as the polarity produced in the molecule by the interaction of two π -bonds or between a π -bond and lone pair of electrons present on an adjacent atom. The effect is transmitted through the chain. It is also known as mesomerie effect. It is a permanent effect.
Negative mesomerie effect: Electron pair is transferred towards the atom or substituent group. It is known as -M effect.
Example : – NO2,-CHO, – SO3H, > C = O.
Positive mesomeric effect: Electron pair is transferred away from an atom or substituent group. It is known as +M effect.
Example : – OH – OR, – SH, – SR, – NH2, – Cl, – Br.
Applications :
- In the determination of structure of benzene.
- In description of dipole moment.
- Capability of acid and base.
- In conjugated addition reactions.
![]()
Question 43.
What are substitution reactions? Explain with example.
Answer:
Reactions in which an atom or group of atoms of a reactant molecule displace atoms or group of atoms of the reagent are called substitution reactions.
Example :
(i) ![]()
Here, H atom of methane is substituted by Cl atom.
(ii) CH3CH2OH + PCl5 → CH3CH2Cl + POCl3 + HCl
In this reaction – OH group is substituted by Cl.
Organic Chemistry: Some Basic Principles and Techniques Class 11 Important Questions Long Answer Type
Question 1.
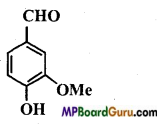
Identify the functional group of the following compounds :
(a)
(b)
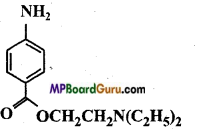
(c)
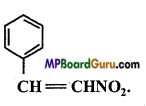
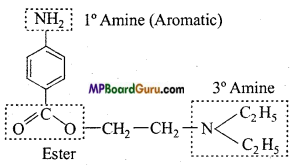
Answer:
(a)
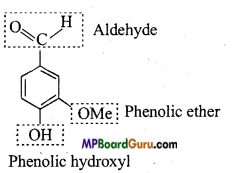
(b)
(c)
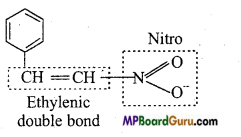
Question 2.
Explain Kjeldahl’s method of estimation of nitrogen under the following points :
1. Principle,
2. Chemical equation,
3. Diagram,
4. Observation and calculation.
Answer:
1. Principle: When nitrogenous organic substance is heated with cone. H2SO4, then nitrogen is completely converted to ammonium sulphate. The reaction mixture when heated with caustic alkali evolves ammonia which is passed in known volume of a standard acid solution.
After the neutralization the volume of remaining acid is determined by titration with a standard solution of an alkali.
2. Chemical equation:
N from organic compound ![]()
(NH4 )2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3
2NH3 + H2SO4 → (NH4)2 SO4
3. Diagram:
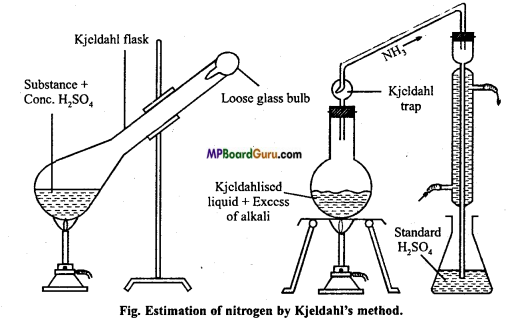
4. Observation and calculation :
Suppose,
- Mass of organic substance =Wgm
- Normality of acid taken initially = N
- Volume of N normal acid taken initially = V1 ml
- Volume of N normal alkali required to neutralize residual acid =V2ml
(This is equivalent to the amount of acid left after neutralization with NH3) Therefore, volume of N normal acid neutralized by NH3
= V1 – V2 = V ml
Because V ml of N normal acid = V ml of N normal alkali = V ml of N normal of NH3 (Acid and base always react in equivalent proportion)
1000 ml of normal solution of NH3 contains =17 gm NH3
= 14 gm nitrogen
∴ Mass of V ml of 1 N NH3 = \(\frac{14}{1000} \) × V gm
∴ Mass of N2 in V ml of N normal NH3 = \(\frac{14}{1000}\) × V × N gm
This weight of nitrogen is present in W gm of substance W gram of substance contains = \(\frac{14 \times \mathrm{V}}{1000} \)
∴ 100 gm substance will contain = \(\frac{14 \times \mathrm{V}}{1000}\) × \( \frac{\mathrm{N} \times 100}{\mathrm{~W}} \) gm nitrogen
= \(\frac{1 \cdot 4 \times V \times N}{W} \)
∴ % of N = \(\frac{1 \cdot 4 \times V \times N}{W}\)
![]()
Question 3.
Which of the two: O2NCH2CH2O or CH3CH2O is expected to be more stable and why?
Answer:
O2NCH2CH2CT is more stable than CH3CH2O“ because (-) NO2 group exhibit -1 effect due to which negative charge is rare. On the other side -CH3 group exhibits +1 effect due to which negative charge becomes dense. Due to less of negative charge stability of ion increases whereas due to dense negative charge stability of ion decreases.
![]()
Question 4.
Give a brief description of the principles of the following techniques taking an example in each case :
(a) Crystallisation,
(b) Distillation,
(c) Chromatography.
Answer:
(a) Crystallisation: In this method, impure compound is converted to pure crystals. It is based on the difference in solubilities of compound and impurities in a suitable solvent. Impure compound is dissolved in such a solvent in which compound is partially soluble at normal temperature, but more soluble at high temperature.
Then the solution is concentrated till saturation. Solution is then cooled when pure compound crystallises. Example: Iodoform crystallises with alcohol. Benzoic acid mixed with naphthalene is purified by hot water.
(b) Distillation: In this method, impure liquid is converted to vapours by heating and again vapours are cooled to convert into liquid. This method is suitable for such liquids which do not decompose on heating and which contain non-volatile impurities. Two such liquids which differ sufficiently in the boiling points can be separated and purified by this method.
Example: Chloroform (b.p. 334K) and aniline (b.p. 457 K) can be easily separated by this method on boiling, liquid of lower b.p. evaporates first which is cooled and collected in the receiver.
(c) Chromatography: It is a technique of separation, purification and identification of components of a mixture. It is based on the specific adsorption of mobile phase of component on stationary phase. Stationary phase can be solid or liquid whereas mobile phase is liquid or gas.
Types of chromatography :
- Column chromatography,
- Thin layer chromatography,
- Paper chromatography.
In column chromatography, stationary phase is solid and mobile phase is a mixture of solvents of variable polarity. Solid adsorbent is taken in a long glass tube with a suitable non-polar solvent. Concentrated solution of the mixture to be separated and purified is passed through upper part of the column. Due to difference in adsorption, the components of the mixture are separated as separated layers known as band (chromatogram).
Question 5.
Explain Victor Meyer method and volumetric method of determination molecular mass of organic compounds.
Answer:
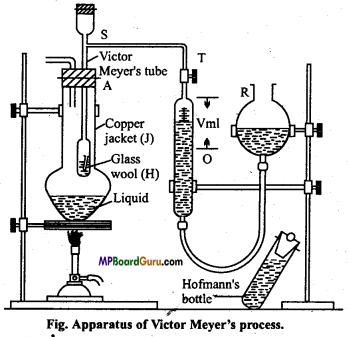
Principle: This method is applicable for determination of mole-cular mass of a volatile substance. A definite mass of organic substance is heated in Victor Meyer’s tube to convert it into vapour state and volume of moist air displaced by vapours is noted. This volume is converted to volume at NTP by further calculation.
Mass of substance which gives 22-4 litre of vapours at NTP is determined. The weight of substance expressed in gram is the molecular mass of substance.
Apparatus: The apparatus used consists of the following parts :
- Copper jacket (J)
- Victor Meyer’s tube (A)
- Hofmann’s bottle.
Calculation: Suppose mass of organic compound = W gram
Volume of dry air displaced at S.T.P. = V ml
Atmospheric pressure = Pmm
Room temperature = t°C or t + 273 K
Aqueous tension at t° C =f
Pressure of dry air = P —f
Volume of vapour at S.T.P. :
In Experimental state At S.T.P.
P1 = P -f
V1 = V ml
T1 = t + 273
P2 = 760
V2 = ?
T2 = 273K
Using gas equation,
\(\frac{P_{1} V_{1}}{T_{1}} \) = \(\frac{\mathrm{P}_{2} \mathrm{~V}_{2}}{\mathrm{~T}_{2}} \)
⇒ V2 = \(\frac{P_{1} \times V_{1} \times T_{2}}{T_{1} \times P_{2}}\)
⇒ V 2 = \(\frac{(P-f) \times V \times 273}{(t+273) \times 760}\)
At S.T.P. V2 ml vapours are obtained from W gm substance.
∴ At S.T.P. 22,400 ml vapours obtained from = \(\frac{\mathrm{W}}{\mathrm{V}_{2}} \) × 22,400 gm.
Molecular mass = \(\frac{\mathrm{W}}{\mathrm{V}_{2}} \) × 22,400 gm.
Question 6.
Describe silver salt method of determination of molecular mass of organic acids.
Answer:
Principle: Organicacid is treated with silver nitrate to form silver salt. These silver salts are heated to obtain silvers. By the help of weight of silver salt and weight of silver, equivalent mass of silver salt is determined. By the equivalent mass of silver salt, equivalent mass and molecular mass of acid can be determined.
Method: Acid is first reacted with excess of NH4OH by which ammonium salt is obtained.
R-COOH+NH4OH → RCOONH4+H2O
It is heated to remove ammonia and neutral solution is treated with silver nitrate to obtain precipitate of silver salt which is washed, dried and its weight is determined.
R- COONH4 + AgNO3 → RCOOAg + NH4NO3
On heating the known weight of silver salt in platinum crucible, silver is obtained whose weight is determined.
Observation and calculation:
Weight of silver salt = Wgm
Weight of silver =x gin
\(\frac{\text { Equivalent weight of silver salt }}{\text { Equivalent weight of silver }}\) = \(\frac{\text { Weight of silver salt }}{\text { Weight of silver }} \)
\(\frac{\text { Equivalent weight of silver salt }}{108}\) = \(\frac{\mathrm{W}}{x}\)
∴ Equivalent weight of silver salt = \(\frac{\mathrm{W}}{x}\) × 108
If E is equivalent weight of acid then on substituting the H by silver, then equivalent weight of silver salt
(E-1) + 108 = E+107
⇒ E + 107 = \(\frac{\mathrm{W}}{x}\) × 108
⇒ E = \(\frac{\mathrm{W}}{x}\) × 108 – 107
If n is the basicity of acid
Molecular mass = [ \(\frac{\mathrm{W}}{x}\) × 108 – 107 ] × n.
Question 7.
Write principle of the chemistry of Lassaigne’s test.
Answer:
Sodium extract: Nitrogen, sulphur and halogens present in organic compound can be detected by Lassaigne’s test. Due to covalent nature of organic compounds, their reaction proceed with slow rate, thus it is impossible to test them directly in the laboratory.
Therefore, they are fused with sodium metal to convert them to ionic compounds and the solution formed is called sodium extract.
Test of Nitrogen:
Test of nitrogen (Lassaigne’s test): In 2 ml of sodium extract, 2 ml of freshly prepared solution of FeSO4 is added along with little NaOH, warm it. Green precipitate of Fe(OH)2 is obtained. The solution is cooled and small quantity of cone. HC1 is added in which green ppt. dissolves. Then little amount of FeCl3 solution is added. If green or blue colour precipitate is obtained, then the substance contains nitrogen.
Na + C + N → NaCN
FeSO4 + 2NaOH → Na2SO4 + Fe(OH )2

Test of sulphur :
(i) Sodium nitroprusside solution is added to the sodium extract of organic substance. If violet colour appears, it confirms the presence of sulphur in the given organic compound.
2Na+S → Na2S

(ii) If acetic acid and lead acetate solution is added to the sodium extract then a black precipitate is obtained, presence of sulphur is confirmed in it.
Na2S + (CH3COO)2 Pb → PbS ↓ Black + 2CH3COONa.
Combined test of Nitrogen and Sulphur :
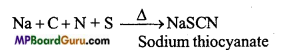
Neutralize the solution with HCl and add FeCl3 solution. Sodium thiocyanate gives blood red colour and does not produce Prussian blue colour like in the test of nitrogen because in this reaction free cyanide ions are not present.
Estimation of halogens: Canus method: In this method estimation of halogens (Cl, Br and I) is done. A known weight of organic compound containing halogen is heated with AgNO3 and fuming nitric acid. Carbon, hydrogen and sulphur present in the compound gets oxidized and halogens form AgX (Silver halide).


Observation:
Mass of Silver halide = Mgm
Mass of organic compound = Wgm
%amount of Cl = \(\frac{35 \cdot 5}{143 \cdot 5}\) × \(\frac{\text { Mass of } \mathrm{AgCl}}{\text { Mass of organic compound }} \) × 100
%amount of Br = \(\frac{80}{188}\) × \(\frac{\text { Mass of } \mathrm{AgBr}}{\text { Mass of organic compound }}\) × 100
% amount of l =\(\frac{127}{235}\) × \(\frac{\text { Mass of AgI }}{\text { Mass of organic compound }}\) × 100.
![]()
Question 8.
Describe chloroplatinate method of determination of molecular mass of organic base.
Answer:
Principle: Organic base reacts with chloroplatinic acid to form insoluble substance of general formula B2H2PtCl6.
B represents equivalent mass of base. By the combustion of platinum salt, metallic platinum is obtained.
![]()
With different masses of platinum salt and platinum, molecular mass of base is determined.
Method: Organic base is first reacted with dilute HCl to obtain soluble hydrochloride. Platinic chloride is mixed in it to obtain salt of the base in the form of chloroplatinate. It is washed and dried. Known weight of it, is heated in platinum crucible to obtain platinum in the crucible as residue. By it equivalent mass of base is determined.
Calculation : Equivalent weight of base = E
Weight of platinum salt = Wgm Weight of platinum = xgm
Molecular mass of B2 H2PtCl6 = 2E + 2 + 195 + 213 = 2 E + 410
\(\frac{\text { Molecular mass of salt }}{\text { Molecular mass of platinum }}\) =\(\frac{\text { Weight of platinum salt }}{\text { Weight of platinum }}\)
or \(\frac{2 \mathrm{E}+410}{195}\) = \(\frac{\mathrm{W}}{x}\)
or 2E = [\(\frac{\mathrm{W}}{x}\) × 195 – 410]
or = \(\frac{1}{2}\) [\(\frac{\mathrm{W}}{x}\) × 195 – 410]
If acidity of base in then,
Molecular mass of base = \(\frac{1}{2}\) [\(\frac{\mathrm{W}}{x}\) × 195 – 410] × n.
Question 9.
Explain Duma’s method of estimation of nitrogen under the following heads:
(i) Principle and method,
(ii) Labelled diagram,
(iii) Observation and calculation.
Answer:
(i) Principle and method: Organic substance and cupric oxide is ignited in hard glass combustion tube in atmosphere of carbon dioxide. Carbon of compound changes to CO2 and hydrogen to H2O. By oxidation nitrogen is obtained in free state. Some oxide of nitrogen are also formed.
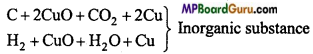
Nitrogen+CuO → N2 + Some amount of some oxides of N2.
Oxides of nitrogen when passed over hot reduced copper gauze change to nitrogen by losing their oxygen.
Oxides of nitrogen + Cu → N2 + CuO
The nitrogen set free is passed through Schiff’s nitrometer filled with 40% solution of caustic potash. Caustic potash solution absorb CO2 while water formed gets condensed. Again CO2 is passed through combustion tube.to drive out all the remaining nitrogen gas to nitrometer. After cooling nitrometer, the volume of nitrogen in nitrometer is noted.
(ii) Labelled diagram :
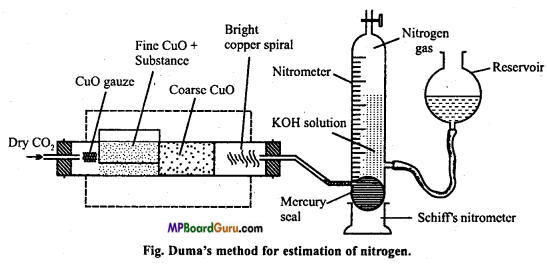
(iii) Observation and calculation
Let,
- Weight of organic compound = W gm
- Volume of moist N = V ml
- Temperature = t°C
- Atmospheric pressure = P mm
- Aqueous tension at t°C = p mm.
Calculation of volume of nitrogen at N.T.P.:
By experiment At N.T.P.
P1 = (P – p) mm
P2 = 760 mm
(Pressure of dry gas)
V, = V ml
V2 = ?
T1 = (t + 273)K
T2 = 0 + 273 = 273K
Using gas equation,
\(\frac{P_{1} V_{1}}{T_{1}}\) = \(\frac{\mathrm{P}_{2} \mathrm{~V}_{2}}{\mathrm{~T}_{2}}\)
\(\frac{(P-p) \times V}{(t+273)}\) = \(\frac{760 \times V_{2}}{273}\)
∴ V2 = \(\frac{(P-p) \times V}{(t+273)}\) × \(\frac{273}{760} \)
∵ Mass of 22400 ml of N2 at N.T.P = 28 gm
∴ Mass of V2 ml of N2 at N.T.P. = \(\frac{28 \times \mathrm{V}_{2}}{22,400}\)
When in W gm of substance N2 is = \(\frac{28 \times V_{2}}{22,400} \times \frac{100}{W}\)
∴ Percentage of N2 in organic compound = \(\frac{28}{22,400} \times \frac{\text { Volume of } \mathrm{N}_{2} \text { at N.T.P. }}{\text { Weight of organic substance }} \times 100\)
Organic Chemistry: Some Basic Principles and Techniques Class 11 Important Numerical Questions
Question 1.
By the combustion of 0.1877gm sample of an organic compound at 14°C and 758mm pressure 31.7ml moist N2 gas is obtained. Calculate the % amount of nitrogen in the compound. (Aqueous tension at 14°C = 12 mm) (NCERT)
Solution: \(\frac{P_{1} V_{1}}{T_{1}}\) = \(\frac{\mathrm{P}_{2} \mathrm{~V}_{2}}{\mathrm{~T}_{2}}\)
P1 = 758 -12mm,
P2 = 760
V1 = 31.7ml
V2 = ?
T1 =14 + 273
T2 = 273
Volume of nitrogen at N.T.P. V2 = \(\frac{(758-12) \times 31 \cdot 7 \times 273}{(14+273) \times 760}\)= 29.6 ml
Percentage amount of N2 =\(\frac{28 \times \mathrm{V}_{2}}{22,400} \times \frac{100}{\mathrm{~W}} \)
= \(\frac{28 \times 29 \cdot 6}{22,400} \times \frac{100}{0 \cdot 1877}\) = 19.72%
Question 2.
0.2870 gram of AgCl is obtained by Carius method by 0.2 gm of an organic compound containing chlorine. Calculate the percentage of chlorine in the compound. (NCERT)
Solution:
Weight of organic compound = 0.2 gm
Weight of AgCl = 0.2870 gm.
∴ Percentage of chlorine = \(\frac{35 \cdot 5}{143 \cdot 5}\) × \(\frac{\text { Weight of } \mathrm{AgCl}}{\text { Weight of organic compound }} \) × 100
= \(\frac{35 \cdot 5}{143 \cdot 5} \times \frac{0 \cdot 2870}{0 \cdot 2} \times 100\) = 35.5%
![]()
Question 3.
In Victor Meyer method 20ml air was displaced over water by the vapours of 0.1gm liquid at 15°C and 765mm pressure. Determine the molecular mass of the liquid (Aqueous tension at 15°C = 13mm).
Solution:
\(\frac{P_{1} V_{1}}{T_{1}}\) = \(\frac{\mathrm{P}_{2} \mathrm{~V}_{2}}{\mathrm{~T}_{2}}\)
P1 = 765 – 13
V1 = 20
T1 = 25 + 273
P2 = 760
V2 = ?
T2 = 273
∴ V2 = \(\frac{P_{1} V_{1} T_{2}}{T_{1} P_{2}}\)
V2 = \(\frac{752 \times 20 \times 273}{288 \times 760}\) = 18.76 ml
∵ Mass of 18.76 ml vapours = 0.1 gm
∴ Mass of 22,480 ml vapours = \(\frac{0 \cdot 1 \times 22,400}{18 \cdot 76}\) = 119.4 gm
Molecular Mass = 119.4 gm
Question 4.
Ammonia produced by the analysis of 06 gm organic compound was absorbed in 90m1 of \(\frac{\mathbf{N}}{\mathbf{9}} \) H2SO4. 20m1 of 01 N caustic soda was required to neutralize the remaining acid. Determine the percent amount of nitrogen in compound. (Atomic mass of H = 1,O = 16, N =14, Na = 23, S = 32) .
Solution:
Acid taken =90 ml \(\frac{\mathbf{N}}{\mathbf{9}} \) H2SO4
= 10 ml NH2SO4
Caustic soda used to neutralize the remaining acid = 20ml 0.1 N NaOH = 2 ml N NaOH
Acid neutralised by ammonia = 10 – 2 = 8 ml (NH4)2SO4
Percent amount of nitrogen = \(\frac{1 \cdot 4 \times N \times V}{W}\)
= \(\frac{1 \cdot 4 \times 1 \times 8}{0 \cdot 6}\)
= 18.66%
Question 5.
In the estimation of sulphur by Carius method, 0.468gm of an organic sulphur compound afforded 0.668gm of barium sulphate. Find out the percentage of sulphur in the given compound. (NCERT)
Solution:
Given, weight of organic compound = 0.468gm.
Weight of BaS04 produced = 0.668gm
Percentage amount of sulphar = \(\frac{32}{233} \) × \(\frac{\text { Weight of } \mathrm{BaSO}_{4} \text { produced }}{\text { Weight of organic compound }} \times 100 \)
= \(\frac{32}{233} \) × \(\frac{0 \cdot 668}{0.468} \) × 100
= 19.60%
Question 6.
An organic substance, A contains C = 40%, H = 6.66% and the rest is oxygen. Vapour density of A is 30. Calculate empirical formula and molecular formula. Or, An organic substance A contains C = 40% and H = 6.66% vapour density of A is 30. It turns blue litmus red and reacts with bases. On heating it, sodium salt with soda lime, first member of paraffin series is obtained, what is it?
Solution:
C = 40%, H = 6.66%
O = 100 -(40 + 6.66)
= 100-46.66 = 53.34%.
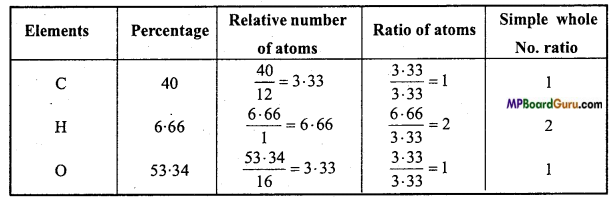
The empirical formula of A = CH2O.
Empirical formula mass = 12 + 2 + 16 = 30
∵ Vapour density of compound = 30
∴ Molecular mass = 2 × V.D. = 2 × 30 = 60
Now, n = \(\frac{\text { Molecular mass }}{\text { Empirical formula mass }}\) = \(\frac{60}{30}\) = 2
∴ Molecular formula of compound = [CH2O]2
= C2H4O2
Substance A turns blue litmus red, therefore it is an acid. On heating its sodium salt with soda lime first member of paraffin series (methane) is obtained therefore the acid will be CH3COOH.

Organic Chemistry: Some Basic Principles and Techniques Class 11 Important Questions Objective Type
1. Choose the correct answer:
Question 1.
Alicyclic compound is:
(a) Aromatic compound
(b) Aliphatic compound
(c) Heterocyclic compound
(d) Aliphatic cyclic compound.
Answer:
(d) Aliphatic cyclic compound.
Question 2.
Chemical formula of dry ice is:
(a) H2O
(b) (H2O)
(c) CO2
(d) H2CO3.
Answer:
(c) CO2
Question 3.
It mainly present in marsh gas:
(a) C2H2
(b) CH4
(c) H2S
(d) CO2.
Answer:
(b) CH4
Question 4.
General formula of alkyne Is:
(a) CnH2n+2
(b) CnH2n+1
(c) CnH2
(d) CnH2n-2
Answer:
(d) CnH2n -2
Question 5.
IUPAC name of (CH3)2 CH-CH = CH – CH3 is:
(a) 2-methylpent-3-ene
(b) 4-methylpent-2-ene
(c) 2-isopropyiprop- 1-ene
(d) 3-isopropylprop-2-ene.
Answer:
(b) 4-methylpent-2-ene
![]()
Question 6.
Chief source of organic compounds is :
(a) Coaltar
(b) Petroleum
(c) Both (a) and (b)
(d) None of these.
Answer:
(c) Both (a) and (b)
Question 7.
What type of isomers are alcohol and ether:
(a) Chain
(b) Position
(c) Functional
(d) Metamerism.
Answer:
(c) Functional
Question 8.
General formula of alcohol is:
(a) CnH2n+1
(b) CnH2n+1.OH
(c) CnH2n-2
(d) CnH2n.
Answer:
(b) CnH2n+1.OH
Question 9.
Which type of isomerism is shown by the following compounds:
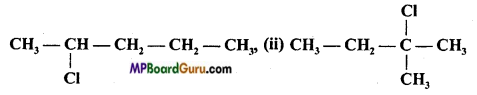
(a) Only functional
(b) Only chain
(c) Position and chain
(d) Only position.
Answer:
(b) Only chain
Question 10.
Which of the following is an example of carbanion:
(a) CH3–
(b)![]()
(c) ![]()
(d) CH3.
Answer:
(b) ![]()
Question 11.
Are known as chiral molecule:
(a) Which do not superimpose on their mirror image
(b) Which superimpose on their mirror image
(c) Which show geometrical isomerism
(d) Which are stable molecules.
Answer:
(a) Which do not superimpose on their mirror image
Question 12.
Isomers are similar in:
(a) Structural formula
(b) Molecular mass
(c) Chemical properties
(d) Physical properties.
Answer:
(b) Molecular mass
![]()
Question 13.
In which of the following all the three groups represent – 1 effect:
(a) -NO2, -Br, -CH3
(b) -I, -NO2, -C2H5
(c) -Cl, -C2H5, -CH3
(d) -F, -NO2, -C6H5.
Answer:
(d) -F, -NO2, -C6H5.
Question 14.
Which of the following compound can exist In geometrical isomer form:
(a) 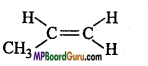
(b) 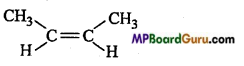
(c) 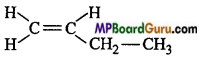
(d) 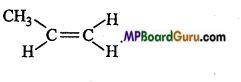
Answer:
(b) 
Question
Example of nucleophile is:
(a) F ion
(b) H3O4ion
(c) Cl atom
(d) Aniline hydrochloride.
Answer:
(a) F ion
Question 16.
ExIstence of a substance In two or more solid forms is known as:
(a) Allotropy
(b) Polymensm
(c) Isomerism
(d) Enantiomerism.
Answer:
(a) Allotropy
Question 17.
Name of compound of structure CI. CH2CH2COOH is:
(a) 3-chioropropanoic acid
(b) 2-chloropropanoic acid
(c) 2-chloroethanoic acid
(d) Chiorosuccinic acid.
Answer:
(a) 3-chioropropanoic acid
Question 18.
Structure of isobutyl chloride is:
(a) CH3CH2CH2CH2Cl
(b) (CH3)2CH.CH2Cl
(c) CH3CH2CHCI.CH3
(d) (CH3)3C-Cl.
Answer:
(c) CH3CH2CHCI.CH3
Question 19.
R-CONH2 is :
(a) An amide
(b) An alcohol
(c) An acid
(d) A cyanide.
Answer:
(a) An amide
![]()
Question 20.
CnH2n is the general formula of:
(a) Alkanes
(b) Alkenes
(c) Alkynes
(d) Arenes.
Answer:
(b) Alkenes
Question 21.
Bond angle in carbon tetrachloride is about:
(a) 90°
(b) 109°
(c) 120°
(d) 180°.
Answer:
(b) 109°
Question 22.
Name of (CH3)2CH-O-CH2-CH2 -CH3 is :
(a) Isopropyl propyl ether
(b) Dipropyl ether
(c) Di-isopropyl ether
(d) Isopropyl propyl ketone.
Answer:
(a) Isopropyl propyl ether
Question 23.
IUPAC name of compound 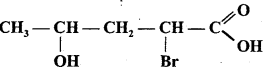 is :
is :
(a) 4-bromo-2-hydroxypentanoic acid
(b) 2-bromo-4-hydroxypentanoic acid
(c) 2-bromo-4-hydroxypentanal
(d) 2-bromo-4-hydroxypentanone.
Answer:
(b) 2-bromo-4-hydroxypentanoic acid
Question 24.
CH3CH2I + KOH(aq) → CH3CH2OH + Kl. Classify this reaction into the following type:
(a) Electrophilic substitution
(b) Nucleophilic substitution
(c) Elimination
(d) Combination.
Answer:
(b) Nucleophilic substitution
Question 25.
General formula of Alkenes Is:
(a) CnH2n+2
(b) Cn H2n
(c) Cn H2n-2
(d) C2n+2Hn.
Answer:
(b) Cn H2n
Question 26.
IUPAC name of the following compound is:
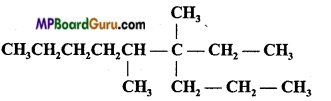
(a) 3, 4-dimethyl-3-n-propylnonane
(b) 6, 7-dimethyl-7-ethyldecane
(c) 6, 7-dimethyl-7-propylnonane
(d) 4, 5-dimethyl-4-ethyLnonane.
Answer:
(d) 4, 5-dimethyl-4-ethyLnonane.
Question 27.
The gas formed by the action of water on calcium carbide is:
(a) Methane
(b) Ethane
(c) Ethylene
(d) Acetylene.
Answer:
(d) Acetylene.
![]()
Question 28.
Baeyer’s reagent is:
(a) Alkaline KMnO4
(b) Ammoniacal AgNO3
(c) Ammoniacal CuSO4
(d) Acidic CaSO4.
Answer:
(a) Alkaline KMnO4
Question 29.
Butane-I, 2-diene contains:
(a) Only sp hybridized carbon atoms
(b) Only sp2 hybridized carbon atoms
(c) sp and sp2 hybridized carbon atoms
(d) sp, sp2 and sp3 hybridized carbon atoms.
Answer:
(d) sp, sp2 and sp3 hybridized carbon atoms.
Question 30.
IUPAC name of compound 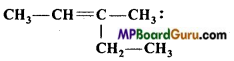
(a) 2-ethylbut-2-ene
(b) 3-ethylbut-2-ene
(c) 3-methylpen-2-ene
(d) 3-methylpent-2-ene.
Answer:
(d) 3-methylpent-2-ene.
Question 31.
IUPAC name of Cl3C.CH2CHO is :
(a) 3, 3, 3-trichloropropanal
(b) 1, 1, l-trichloropropanal
(c) 2, 2, 2-trichloropropanal
(d) Chloral.
Answer:
(a) 3, 3, 3-trichloropropanal
Question 32.
Three-dimensional isomers differ in :
(a) Configuration
(b) Conformation
(c) They are not different
(d) None of these.
Answer:
(a) Configuration
Question 33.
CH3 -CH(OH) – COOH represent:
(a) Geometrical isomerism
(b) Optical isomerism
(c) Both (a) and (b)
(d) None of these.
Answer:
(b) Optical isomerism
Question 34.
Nitroethane is one of the type of the following isomerism :
(a) Metamerism
(b) Optical activity
(c) Tautomerism
(d) Position isomerism.
Answer:
(c) Tautomerism
Question 35.
Aniline is generally purified by :
(a) Steam distillation
(b) Simple distillation
(c) Distillation under reduced pressure
(d) Sublimation.
Answer:
(a) Steam distillation
Question 36.
Glycerol boils at 290°C with slight decomposition. Impure glycerol is purified by:
(a) Steam distillation
(b) Simple distillation
(c) Vacuum distillation
(d) Extraction with solvent.
Answer:
(c) Vacuum distillation
![]()
Question 37.
The blue or green colour obtained in Lassaigne’s test is due to the formation of:
(a) NaCN
(b) Na4[Fe(CN)6]
(c) Fe3[Fe(CN)6]4
(d) Fe4[Fe(CN)6]3.
Answer:
(d) Fe4[Fe(CN)6]3.
Question 38.
Formation of blood-red colouration in Lassaigne’s test indicates the presence of:
(a) Nitrogen
(b) Sulphur
(c) Nitrogen and sulphur
(d) Sulphur and iodine.
Answer:
(b) Sulphur
Question 39.
The blood-red coloured compound formed during the qualitative analysis of nitrogen and sulphur together is :
(a) Fe4[Fe(CN)6]2
(b) Fe(SCN)3
(c)KSCN
(d) Na2S.NaCN.
Answer:
(b) Fe(SCN)3
Question 40.
Kjeldahl method is used for estimation of:
(a) Sulphur
(b) Nitrogen
(c) Halogens
(d) Oxygen.
Answer:
(c) Halogens
Question 41.
A compound with empirical formula CH2O has molecular mass 90. The formula of compound is:
(a) C4H10O2
(b) C2H5O
(c) C3H6O3
(d) C5H14O.
Answer:
(c) C3H6O3
Question 42.
The amount of sulphur present in an organic compound is estimated by changing it into:
(a) H2S
(b) SO2
(c) H2SO3
(d) H2SO4.
Answer:
(d) H2SO4.
![]()
Question 43.
The reagent used in Carius method to estimate halogens is :
(a) HNO3 and HCl
(b) HNO3 and H2SO4
(c) Fuming HNO3 and BaCl2
(d) Fuming HNO3 and AgNO3.
Answer:
(d) Fuming HNO3 and AgNO3.
Question 44.
The gas collected in Duma’s method of estimation of nitrogen in organic com-pound is:
(a) N2
(b) NO
(c) NH3
(d) None of these.
Answer:
(a) N2
Question 45.
An organic compound contains C = 80% and H = 20%. The compound shall be:
(a) C6H6
(b) C2H5OH
(c) C2H6
(d) CHCl3.
Answer:
(c) C2H6
Question 46.
An organic compound contains C = 39-9%, H = 6-7%, O = 53*4%. Its empirical formula will be:
(a) CHO
(b) CHO2
(c) CH2O2
(d) CH2O.
Answer:
(d) CH2O.
Question 47.
In an organic compound, the ratio of mass is C: H: O = 4: 1: 5. Its empirical formula shall be:
(a) C2HO
(b) C5H4O4
(c) CH4O2
(d) CH3O.
Answer:
(d) CH3O.
Question 48.
The best modern technique of separation and purification of organic compounds is :
(a) Crystallization
(b) Distillation
(c) Sublimation
(d) Chromatography.
Answer:
(d) Chromatography.
Question 49.
In the organic compound CH2 = CH – CH2 – CH2 – C = CH, by which hybrid orbital C2– C3 bond is formed :
(a) sp – sp2
(b) sp – sp3
(c) sp2 – sp3
(d) sp3 – sp3.
Answer:
(c) sp2 – sp3
Question 50.
Formation of Prussian blue colour for the identification of nitrogen by Lassaigne’s test in an organic compound is obtained due to :
(a) Na4[Fe(CN)6]
(b) Fe4[Fe(CN)6]3
(c) Fe2[Fe(CN)6]
(d) Fe3[Fe(CN)6]4.
Answer:
(b) Fe4[Fe(CN)6]3
Question 51.
Which of the following carbocation is most stable :
(a) ![]()
(b) ![]()
(C) ![]()
(d) ![]()
Answer:
(b) ![]()
2. Fill in the blanks:
1. IUPAC name of 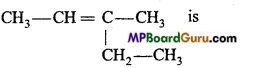 is ………………….. .
is ………………….. .
Answer:
3-methylpent-2-ene
2. Chemical name of freon organic compound which is used in air conditioner and refrigerators is………………….. and its chemical formula is ………………….. .
Answer:
Chlorofluoro carbon,CF2Cl2
3. ………………….. ratio of elements in a compound is called its empirical formula.
Answer:
Simplest
4. The process of fractional crystallization of separation of two substances depend on the difference of ………………….. .
Answer:
Solvent
5. In organic compound, presence of amount of halogen can be detected by converting it into ………………….. .
Answer:
Silver halide
6. A compound contains 80% carbon and 20% hydrogen, its formula will be ………………….. .
Answer:
C2H6
7. Marsh gas mainly contains ………………….. .
Answer:
Methane
8. Process of separation of substances at different rates of adsorption is known as ………………….. .
Answer:
Elution
9. Kjeldahl method is used for the estimation of …………………… element.
Answer:
Nitrogen
10. Separation of mixture of two substances is based on ………………….. .
Answer:
Solubility
11. Separation of mixture of o-nitrophenol and p-nitrophenol is based on ………………….. .
Answer:
Steam distillation
12. Glycerine decomposes at its boiling point, it is purified by ………………….. .
Answer:
Distillation at low pressure.
![]()
3. Match the following:
| ‘A’ | ‘B’ |
| 1. Marsh gas | (a) (CH3)-C-OH |
| 2. Vinegar | (b) CH3-CH2-OH |
| 3. Absolute alcohol | (c) HCHO |
| 4. Formalin | (d) CH3NC |
| 5. lodoform | (e) 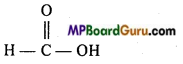 |
| 6. Ethane nitrile | (f)  |
| 7. Carbylamine methane | (g) CH3-COOH |
| 8. Formic acid | (h) CH4 |
| 9. Gem dihalide | (i) CHl3 |
| 1o. Tertiary butyl alcohol | (j)CH3CN. |
Answer:
1. (h) CH4
2. (g) CH3-COOH
3. (b) CH3-CH2-OH
4. (c) HCHO
5. (j) CH3CN
6. (i) CHl3
7. (d) CH3NC
8. (e) 
9. (f) 
10. (a) (CH3)-C-OH.
4. Answer in one word/sentence:
1. IUPAC name of CH3 – CO – CH3 is.
Answer:
Propanone-2
2. Chemical name of CH3 – CH2 – CHCl – CH3 is.
Answer:
Iso butyl chloride
3. Mixture of KMnO4 and KOH is known as.
Answer:
Baeyer’s reagent
4. On adding femc chloride in sodium extract red colour is obtained which prove the presence of which element?
Answer:
Nitrogen and sulphur
5. In organic compounds amount of S is determined by converting it to which compound?
Answer:
H2SO
6. How many moles of H2O are formed by the burning of one mole C3H6 in air?
Answer:
6
7. IUPAC name of Vinegar is.
Answer:
Ethanoic acid
8. IUPAC name of çthyl alcohol is.
Answer:
Ethanol
9. Camphor is separated from a mixttire of NaCl is?
Answer:
Sublimation
10. Naphthalene is purified by.
Answer:
Sublimation
11. Separation by coLumn chromatography is due to.
Answer:
Difference in adsorption
12. Petroleum is refined by.
Answer:
Fractional distillation
13. Beilstein Test is used.
Answer:
In detection of halogens
14. Free radicals are formed due to which type of fission?
answer:
Homolytic fission.