MP Board Class 8th Science Solutions Chapter 6 Combustion and Flame
MP Board Class 8th Science Combustion and Flame NCERT Textbook Exercises
Mp Board Class 8 Science Chapter 6 Question 1.
List conditions under which combustion can take place.
Answer:
Conditions for combustion are:
- The substance must be combustible.
- Air is necessary for combustion.
- The temperature of the substance to be burnt should be higher than its ignition temperature.
- It is essential for a substance to reach ignition temperature to bum.
Mp Board Class 8 Science Solution Chapter 6 Question 2.
Fill in the blanks:
(a) Burning of wood and coal causes ………… of air.
(b) A liquid fuel, used in homes is …………..
(c) Fuel must be heated to its …………….. before it starts burning.
(d) Fire produced by oil cannot be controlled by……………..
Answer:
(a) pollution
(b) kerosene oil
(c) ignition temperature
(d) water.
Class 8 Science Chapter 6 Mp Board Question 3.
Explain how the use of CNG in automobiles has reduced pollution in our cities?
Answer:
The use of CNG in automobiles has reduced pollution in our cities because CNG does not produce any poisonous gases on burning. It is less polluting and a cleaner fuel.
Mp Board Class 8 Social Science Solution Chapter 6 Question 4.
Compare LPG and wood as fuels.
Answer:
Comparison between LPG and wood as fuels:

Mp Board Class 8 English Chapter 6 Question 5.
Give reasons:
(a) Water is not used to con retrofires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminum pipe does not.
Answer:
(a) Water is not used at all to control the tire involving electrical equipment because water is a good conductor of electricity and it may result in electric shock or electrocution.
(b) LPG is a better fuel than wood because LPG does not produce harmful smoke like wood. It does not pollute the environment. It is easy to store and handle.
(c) The ignition temperature of paper is higher than that of a piece of paper wrapped around an aluminum pipe. 6. Make a labeled diagram of a candle flame.
Mp Board Class 8th Science Chapter 6 Question 6.
Make a labeled diagram of a candle flame
Answer:
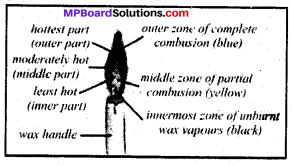
Mp Board Class 8 Social Science Chapter 6 Question 7.
Name the unit in which the calorific value of a fuel is expressed.
Answer:
It is expressed in kilo joules per kg. (kJ/kg).
Combustion And Flame Class 8 Question 8.
Explain how CO2 is able to control fires.
Answer:
Carbon dioxide cuts off the supply of air to the combustible substance and extinguishes fire. Also CO2 being heavier than oxygen, covers the fire like a blanket. Since the contact ‘between The fuel and oxygen L cut on, -ha. fire is controlled. The added advantage of CO2 is that in most cases it does not harm the electrical equipment.
Class 8 Science Chapter 6 Question Answer Question 9.
It is difficult to bum a heap of green leaves but dry leaves catch fire easily. Explain.
Answer:
Green leaves contain lot of water. So, when we try to bum them, water contained in the leaves cools the combustible materials (leaves), so that its temperature is brought below its ignition temperature. This prevents the burning of green leaves. In case of dry leaves, they do not contain any water. So when burning process starts, its temperature is raised drastically above its ignition temperature and the leaves catch fire easily.
Class 8 English Chapter 6 Mp Board Question 10.
Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer:
- The goldsmith uses the outermost zone of a flame with a metallic blow-pipe for melting gold and silver.
- The flame in the outer most zone has. the highest temperature sufficient to melt the gold and silver.
Combustion And Flame Class 8 Notes Pdf Question 11.
In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kj. Calculate the calorific value of the fuel.
Answer:
We know that,
Calorific value fuels
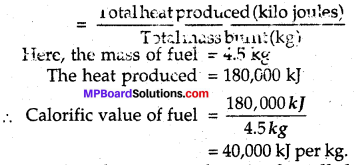
Class 8th Science Chapter 6 Exercise Question 12.
Can the process of rusting be called combustion? Discuss.
Answer:
The process of rusting cannot be called combustion because neither energy is released nor heat and light are produced during it, while in combustion-release of energy takes place with heat and light.
Class 8 Science Chapter 6 Question 13.
Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water still get heated in a shorter time?
Answer:
Ramesh’s water will get heated in a shorter time because the outermost part of the flame is the hottest.
MP Board Class 8th Science Combustion and Flame Extended Learning – Activities and Projects
Mp Board Class 8 Science Book Pdf Question 1.
Survey the availability of various fuels in your locality. Find out their cost per kg and prepare a tabular chart showing how many kJ of various fuels you can get for every rupee.
Answer:
Various fuels like LPG, kerosene, cow dung.
LPG for ? 431 for 14.2 kg.
Kerosene oil ? 20 for one liter.
Cow dung for ? 5 for one kg.
Question 2.
Find out the number, type and location of fire extinguishers available in your school, nearby shops and factories. Write a brief report about the prepareness of these establishments to fight fire.
Answer:
Do yourself.
Question 3.
Survey 100 houses in your area. Find the percentage of households using LPG, kerosene, wood and cattle dung as fuel.
Answer:
Do yourself.
Question 4.
Talk to people who use LPG at home. Find out what precautions they take in using LPG.
Answer:
The following precautions are taken:
- They keep their cylinders in standing position.
- They switch off regulators after the use.
- They keep on checking and cleaning pipe time to time.
Question 5.
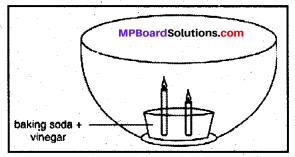
Make a model of a fire extinguisher. Place a short candle and a slightly taller candle in a small dish filled with baking soda. Place the dish at the bottom of a large bowl. Light both the candles. Then pour vinegar into the dish of baking soda. Take care. Do not pour vinegar on the candles. Observe the foaming reaction. What happens to the candles? Why? In what order?
Answer:
The candles get extinguished. The smaller candle will get extinguished first because the supply of oxygen is cut off due to foam. The smaller candle will come in effect of foam earlier than the longer one and thus stop burning prior to the longer candle.
MP Board Class 8th Science Combustion and Flame Intext Activities and Projects
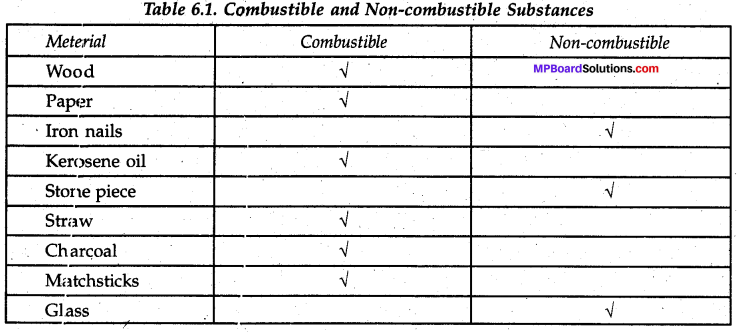
Collect some materials like straw, matchsticks, kerosene oil, paper, iron nails, stone pieces, glass etc.
Under the supervision of your teacher try to burn each of these materials one by one. If combustion takes place mark the material combustible, otherwise mark it as non-combustible (Table 6.1).
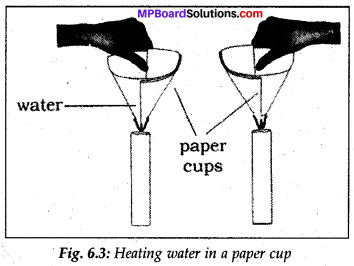
Make two paper cups by folding a sheet of presence of water, the ignition temperature paper. Pour about 59 mL of water in one of Df paper is not reached. Hence, it does not the cups. Heat both the cups separately with burn. a candle (Fig. 6.3) What do you observe?
What happens to the empty paper cup?
What happens to the paper cup with water?
Does water in this cup become hot?
Answer:
If we continue heating the cup, we can even boil water in the paper cup but the empty. Cup when heated shall burn.
Can you think of an explanation for this phenomenon?
The heat supplied to the paper cup is transferred to water by conduction. So, in the presence of water, the ignition temperature of paper is not reached. Hence, it does not burn.
Record your observations and mention whether on burning the material forms a flame or not.
TABLE 6.2 Materials forming Flame on Burning
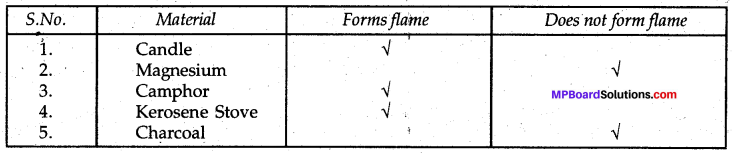
TABLE 6.3 Types of Fuels
Make a list of fuels familiar to you. Group them as solid, liquid arid gaseous fuels as in table.

MP Board Class 8th Science Combustion and Flame Additional important Questions
A. Short Answer Type Questions
Question 1.
What is combustion? Name three combustible substances.
Answer:
Combustion is the process of burning of a substance in the presence of oxygen to liberate energy in the form of heat and light. Combustible substances are wood, paper and kerosene.
Question 2.
Can you name few fuels used in our homes?
Answer:
Cowdung, wood, coal, charcoal, kerosene and LPG are few fuels used in our homes.
Question 3.
Name few fuels used in trade and industry.
Answer:
LPG, coal, petrol, diesel and nuclear fuels are used in trade and industry.
Question 4.
Can you burn a piece of wood by bringing a lighted matchstick near it?
Answer:
No, because wood has high ignition temperature and it requires to be heated for longer time to start its burning. Whereas the lighted matchstick extinguishes very soon.
Question 5.
Why do you have to use paper dr kerosene oil to start fire in wood or coal?
Answer:
As wood or coal has high ignition point, it requires a lot of time to be heated before burning can take place. That is why paper or kerosene oil are burnt near wood to start fire.
B. Long Answer Type Questions
Question 6.
What are different types of combustion?
Answer:
Combustion is of three types:
- Rapid Combustion: When gases bum
- Spontaneous Combustion: When any material like phosphorus bums on its own without any apparent cause, it is called spontaneous combustion.
- Explosion: When combustion takes place with sudden release of heat and light and a large amount of gas in form of bang, it is called explosion as in case of crackers and bombs.
Question 7.
Mention three characteristic features of an ideal fuel.
Answer:
- An ideal fuel is cheap, readily available, readily combustible and easy to transport.
- It has high calorific value.
- It does not produce gases or residues that pollute the environment.
Question 8.
Write a short note on fire extinguinavishers.
Answer:
In most of the fire extinguishers, carbon dioxide gas is used for extinguishing fire. Carbon dioxide, being heavier than air surrounds the burning substance and disrupts the supply of oxygen. Carbon dioxide neither bums nor helps in burning and therefore, fire gets extinguished. In soda acid extinguishers, a mixture of CO2 and water is sprayed over fire. Water reduces the ignition temperature of the burning substance and C02 disrupts supply pf oxygen thereby fire is extinguished.