MP Board Class 8th Science Solutions Chapter 14 Chemical Effects of Electric Current
MP Board Class 8th Science Chemical Effects of Electric Current NCERT Textbook Exercises
Question 1.
Fill in the blanks:
(a) Most liquids that conduct electricity are solutions of ……………, ……………. and ………….
(b) The passage of an electric current through a solution causes ……………. effects.
(c) If you pass current through copper sulphate solution, copper gets deposited on the plate connected to the ………. terminal of the battery.
(d) The process of depositing a layer of any desired metal on another material by means of electricity is called ………..
Answer:
(a) acids, bases, salts
(b) chemical
(c) negative
(d) electroplating.
Question 2.
When the free ends of a tester are
dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
Answer:
Yes, the solution conducts electricity.
Question 3.
Name three liquids, which when tested in the manner shown in Fig 14.1 may cause the magnetic needle to deflect.
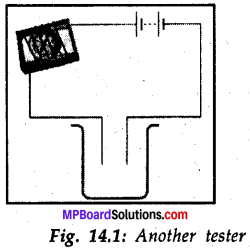
Answer:
Tap water, lime water, vinegar or lemon juice.
![]()
Question 4.
The bulb does not glow in the setup shown in Fig. 14.2. List the, possible reasons. Explain your answer.
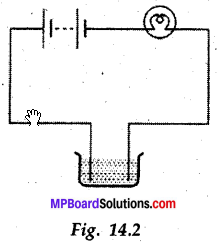
Answer:
If the bulb does not glow it may be because of some reasons like the bulb may be fused. Replaced with a new bulb, if it still does not glow, it shows that the connection of wires may be loose. After tightening connections if still the bulb does not glow, then it is for sure that the solution does not conduct electric current.
Question 5.
A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightly for liquid Awhile it glows very dimly for liquid B. You would conclude that:
(i) Liquid A is a better conductor than liquid B.
(ii) Liquid B is abeHer conductor than liquid A.
(iii) both liquids are equally conducting.
(iv) conducting properties of liquid cannot be compared in this manner.
Answer:
(i) Liquid A is a better conductor than liquid B.
Question 6.
Does pure water conduct electricity? If not, what can we do to make it conducting?
Answer:
Pure water does not conduct electricity. To make it conducting we may add to it a little common salt or acid or a base.
Question 7.
In case of any fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain the reason why they do this?
Answer:
If an electric wire or appliance becomes wet, the risk of electrocution increases. Therefore, to ensure safety, the firemen shut off the main electrical supply for the area to extinguish fire due to electric short circuit.
Question 8.
A child staying in the coastal region tests the drinking water and also the sea-water with his tester. He finds that the compass needle deflects more in case of sea-water. Can you explain the reason?
Answer:
Sea-water is better conductor than the drinking water because sea-water contains mineral salts dissolved in it. It is therefore, the compass needle deflects more in case of seawater.
Question 9.
Is it safe for the wireman to carry out electrical repairs during heavy downpour? Explain.
Answer:
No, During heavy downpour, there is a risk of electrocution because impure water is good conductor of electricity.
![]()
Question 10.
Pooja knew that rainwater is as pure as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise she found that the compass needle showed deflection. What could be the reasons?
Answer:
Rainwater is, of course, as good as distilled water but when it passes through atmosphere, it dissolves a lot of dust, dirt and impurities and becomes conducting. So, when Pooja used a tester, its compass showed deflection.
Question 11.
Prepare a list of objects around you that are electroplated.
Answer:
The objects which are electroplated are:
- Handles and rims of a bicycles
- Utensils of kitchen
- Metallic showpieces
- Metallic containers, buckets
- bath tubs
- Artificial jewelery
- kitchen gas burner.
Question 12.
The process that you saw in Activity 14.7 (NCERT Textbook page no. 178) is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of battery and why?
Answer:
Impure copper rod should be attached to the positive terminal of the battery in order to make it anode. This will enable the deposition of copper on thin copper plate which acts as a cathode.
MP Board Class 8th Science Chemical Effects of Electric Current NCERT Extended Learning – Activities
Question 1.
Test the conduction of electricity through various fruits and vegetables, Display your result in a tabular form.
Answer:
Do yourself.
Question 2.
Repeat the Activity 14.7 (Textbook page no. 178) with a zinc plate in place of the copperplate connected to the negative terminal of the battery. Now replace zinc plate with some other metallic object and again repeat the activity. Which metal gets deposited over which other metal? Discuss your findings with your friends.
Answer:
Do yourself.
Question 3.
Find out if there is a commercial electroplating unit in your town. What objects are electroplated there and for what purpose? (The process of electroplating in a commercial unit is much more complex than what we did in Activity 14.7 of textbook. Find out how they dispose off the chemicals they discard.
Answer:
Do yourself.
![]()
Question 4.
Imagine that you are an ‘entrepreneur’ and have been provided a loan by a bank to set up a small electroplating unit. What object you would like to electroplate and for what purpose? (Look meaning of ‘entrepreneur’ in a dictionary).
Answer:
Entrepreneur is a person who makes money by starting or running business, especially when this involves taking financial risks.
Question 5.
Find out the health concerns associated with chromium electroplating. How are people trying to resolve them?
Answer:
Do yourself.
Question 6.
You can make a fun pen for yourself. Take a conducting metal plate and spread a moist paste of potassium iodide and starch. Connect the plate to a battery as shown in Fig. 14.3. Now using the free end of the wire, write a few letters on the paste. What do you see?
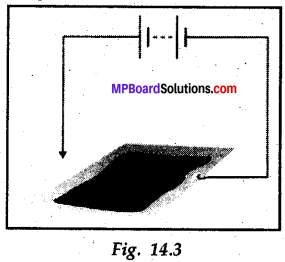
Answer:
Do yourself.
MP Board Class 8th Science Chemical Effects of Electric Current NCERT Additional Important Questions
A. Short Answer Type Questions
Question 1.
What types of forces do electric charges exert on each other?
Answer:
Unlike charges attract each other referred to as an electric circuit, while like charges repel each other.
Question 2.
What is an electric circuit?
Answer:
The path of an electric current is referred to as an electric circuit.
![]()
Question 3.
How can we check magnetic effects of electric current?
Answer:
We can check magnetic effects of electric current by using magnetic compass.
Question 4.
What is used in CFLs?
Answer:
Mercury is used in CFLs.
Question 5.
Why is it very dangerous to operate an electrical appliance with wet hands?
Answer:
If someone operates electrical appliances with wet hands, there is a chance of getting electrocuted.
B. Long Answer Type Questions
Question 6.
What effect does the current produce when it flows through a conducting solution?
Answer:
The passage of an electric current through a conducting solution causes chemical reactions. As a result, bubbles of gas are formed, deposits of metal on electrodes may be seen and changes of colour of solution may occur, depending on what solutions and electrodes are used. These are some of the chemical effects of the electric current.
Question 7.
What are the different effects of electricity?
Answer:
The different effect of electricity are:
- Heating effect
- Chemical effect
- Magnetic effect.
Question 8.
What is the application of chemical effects of electricity in our daily life?
Answer:
Carrying chemical reactions by the effect of electricity is called chemical effect of electric current.
![]()