MP Board Class 6th Science Solutions Chapter 5 Separation of Substances
Separation of Substances Text Book Exercises
Question 1.
Why do we need to separate different components of a mixture? Give two examples?
Answer:
It is essential to purify foodstuff to maintain good health. Researchers, chemists and technologists also need pure substances. Thus, separation of different components of a mixture is essential. The main purposes are:
- To remove impurities for getting a pure sample. For example, salt from sea water.
- To obtain the useful components for getting wheat or rice grains after separating.
- To remove the harmful component.
![]()
Question 2.
What is winnowing? Where it is used?
Answer:
The process of separating heavier and lighter components of a mixture by wind or by blowing air is called winnowing? This method is commonly used by farmers to separate lighter husk particles from heavier seeds of grain. The farmer allows the mixture of grain and the husk to fall from a height.
The grains which are heavier fall vertically down on the ground and form a heap near the platform of winnowing. The husk which is higher, is carried away by the wind and forms a separate heap at a short distance from the heap of grains as shown in the figure.

The separated husk is used for many purposes such as fodder for catels.
Question 3.
How will you separate husk or dirt particles from a given sample of pulses before cooking?
Answer:
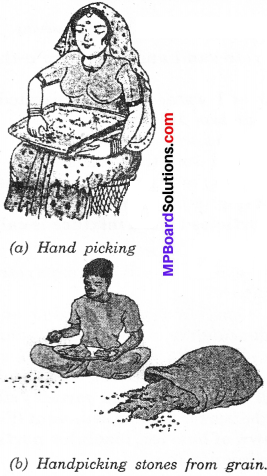
The husk or dirt particles from a given sample of pulses before cooking can be separated by hand picking. For example, when the components of a mixture are different in size, shape and color, they can be easily separated by hand picking. Housewife often clean pulses and spices by this method.
They remove small dust, dirt, pebbles and other unwanted materials. The quantity of such impurities is usually not very large. In such situations, we find that hand picking is a convenient method of separating substances.
Question 4.
What is sieving? Where is it used?
Answer:
Sieving is a method used to separate the components of a mixture which are of different size. Sieving allows the fine flour particles to pass through the holes of the sieve while the bigger impurities remain on the sieve In a flour mill, impurities like husk and stones are removed from wheat before grinding it.
Usually, a bagful of wheat is poured on a slanting sieve. The sieving removes pieces of stones, stalk and husk that may still remain with wheat after threshing and winnowing. You may have also noticed similar sieves being used at construction sites to separate pebbles and stones from sand.


Question 5.
How will you separate sand and water from their mixture?
Answer:
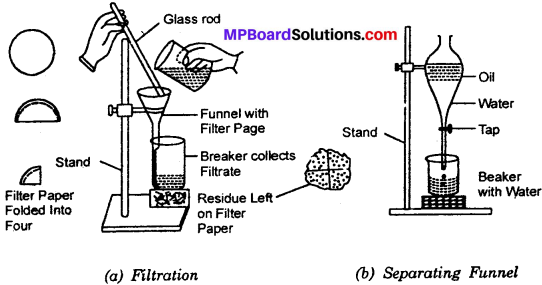
A mixture of sand and water can be separated by sedimentation method or decantation method. Take the mixture of sand and water in a beaker. Allow the mixture to stand for sometime undisturbed.
You will observe that sand particles start settling down at the bottom of the beaker forming sediment (Fig. (a). The process of settling down of heavier, insoluble particles from a mixture is called sedimentation.
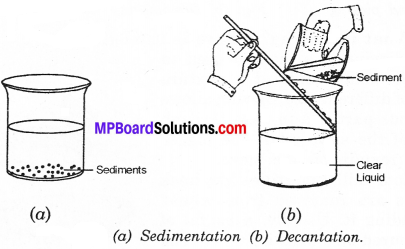
The clear liquid above the sediment is gently poured off into another beaker, without disturbing the sediment (Fig. b). This process of transferring the clear liquid without disturbing sediments is known as decantation.
Question 6.
Is it possible to separate sugar mixed with wheat flour? If yes, how will you do it?
Answer:
Yes, it is possible to separate sugar mixed with wheat flour. This can be separated by sieving. Sieving allows the fine wheat flour particles to pass through the holes of the sieve while the sugar remain on the sieve.
![]()
Question 7.
How would you obtain clear water from a sample of muddy water?
Answer:
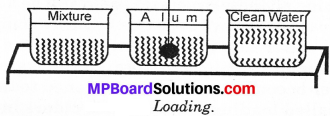
We can separate clear water from a sample of muddy water by loading process. Take a beaker half filled with muddy water and take a piece of alum. The loading of suspended particles is carried out by alum. The alum crystal is slowly moved in water.
The dissolved particles of alum in water loads on the fine dust particles. The particles become heavy and settles down to the bottom. The clear water is decanted as shown in Fig. The suspended particles remains at bottom as sediment.
Question 8.
Fill up the blanks:
- The method of separating seeds of paddy from its stalks is called ………………………
- When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example ……………………………..
- Salt is obtained from seawater by the process of ……………………….
- Impurities settled at the bottom when muddy water was kept overnight in a bucket. The clear water was then poured off from the top. The process of separation used in this example is called ………………………..
Answer:
- Threshing
- Filtration
- Evaporation
- Decantation.
Question 9.
True or false:
- A mixture of milk and water can be separated by filtration.
- A mixture of powdered salt and sugar can be separated by the process of winnowing.
- Separation of sugar from tea can be done with filtration.
- Grain and husk can be separated with the process of decantation.
Answer:
- False
- False
- False
- False.
![]()
Question 10.
Lemonade is prepared by mixing lemon juice and sugar in water. You wish to add ice to cool it. Should you add ice to the lemonade before or after dissolving sugar? In which case would it be possible to dissolve more sugar?
Answer:
We should add ice in lemonade after dissolving sugar in it, because sugar dissolve more quickly before adding ice. It would be possible to dissolve more sugar before adding ice in the lemonade, because it dissolve more into hot than in cold.
Projects and Activities
Activity 1.
You have tried a number of methods to separate impurities like mud from water. Sometimes, the water obtained after employing all these processes could still be a little muddy. Let us see if we can remove even this impurity completely. Take this filtered water in a glass?
Tie a thread to a small piece of alum. Suspend the piece of alum in the water and swirl. Did the water become clear? What happened to the mud? This process is called loading. Talk to some elders in your family to find out whether they have seen or used this process?
Answer:
We can separate clear water from a sample of muddy water by loading process. Take a beaker half filled with muddy water and take a piece of alum. The loading of suspended particles is carried out by alum. The alum crystal is slowly moved in water.
The dissolved particles of alum in water loads on the fine dust particles. The particles become heavy and settles down to the bottom. The clear water is decanted as shown in Fig. The suspended particles remains at bottom as sediment.

Activity 2.
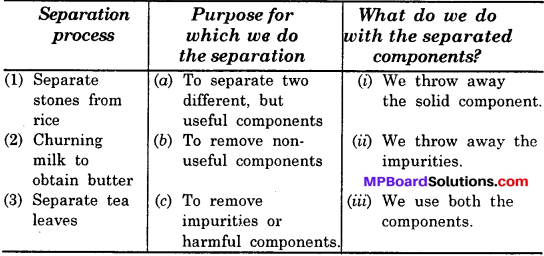
Match the each process with its purpose and the way separated components are used:
Table: Why do we separate substances?
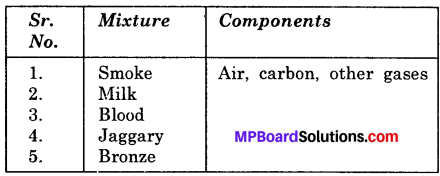
Activity 3.
Complete the following table:
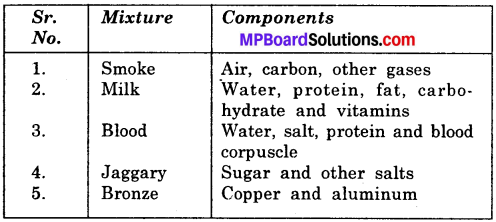
Answer:
Activity 4.
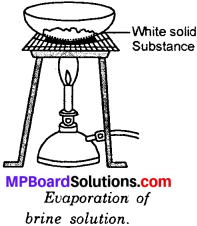
To separate common salt from common salt solution (brine)?
Method:
Take brine in a china dish. Heat it by placing it over a wire gauze on a tripod stand as shown in Fig. After sometime the water starts evaporating. Go on heating till all the water present in the solution evaporates. You will see that some white solid substance is left behind as a residue. This is a sample of pure common salt.
Activity 5.
To separate a mixture of kerosene and water?
Method:
Prepare a mixture of kerosene and water in a 200ml. Beaker. Carefully transfer to a separating funnel through a funnel. Stopper and shaken well. Now the mixture allow to settle. Note that kerosene is the top layer and water is the bottom layer.
This is due to the density differences between the two liquids Place the separating funnel in a ring stand and place a beaker below it to collect the components. Carefully looking at the layers of separation, drain kerosene and water in different containers.
Separation of Substances Additional Important Questions
Separation of Substances Objective Type Questions
Question 1.
Choose the correct answer:
Question (a)
A mixture of iodine and sand can be separated by –
(a) Decantation
(b) Centrifugation
(c) Filtration
(d) Sublimation.
Answer:
(d) Sublimation.
Question (b)
A mixture of leaves of tea and iron fillings are separated by –
(a) Filtration
(b) Handpicking
(c) Magnetic separation
(d) Sieving.
Answer:
(c) Magnetic separation
Question (c)
A mixture of mustard oil and kerosene oil can be separated by –
(a) Sublimation
(b) Evaporation
(c) Separating funnel
(d) Filtration.
Answer:
(c) Separating funnel
![]()
Question (d)
When two elements are brought together, the always form
(a) A mixture
(b) An element
(c) A compound
(d) None of these.
Answer:
(a) A mixture
Question (e)
In nature most elements occur as –
(a) Pure elements
(b) Mixture of elements
(c) Compound form
(d) None of these.
Answer:
(c) Compound form
Question (f)
A solution of salt in water is a –
(а) Compound
(b) Homogeneous mixture
(c) Heterogeneous mixture
(d) None of these.
Answer:
(b) Homogeneous mixture
Question 2.
Fill in the blanks:
- A mixture of mustard oil and water can be separated by using ………………………
- Ammonium chloride and salt are separated by …………………………
- Common salt is obtained from sea water by …………………….
- A mixture of chalk powder and water is seperated by ………………………
- Husk is separated from rice by …………………………
- Cream is separated from milk by ……………………….
- Naphthalene is separated from common salt by ……………………..
- Insects are separated from wheat by ……………………..
Answer:
- Separating funnel
- Sublimation
- Evaporation
- Filtration
- Winnowing
- Centrifugation
- Sublimation
- Hand – picking.
![]()
Question 3.
Which of the following statements are true (T) or false (F):
- Sherbet is a pure substance.
- Rock salt is impure substance.
- The process of winnowing is used to remove small pieces of stone from grams.
- A pure sample of a substance consists of only one kind of particles.
- Sugar is separated from its solution in water by decantation.
- Butter is separated from milk by the. method of crystallization.
Answer:
- False
- True
- False
- True
- False
- False.
Question 4.
Match the items in Column A with the Column B:

Answer:
(i) – (c)
(ii) – (a)
(iii) – (b)
(iv) – (d).
Separation of Substances Very Short Answer Type Questions
Question 1.
Name the process used in flour mill?
Answer:
Sieving.
Question 2.
Name the method of separating iron from waste material?
Answer:
Magnetic separation.
Question 3.
Name the process to separate two unmixible liquids?
Answer:
Decantation.
Question 4.
Name the apparatus used to separate two unmixible liquids?
Answer:
Separating funnel.
![]()
Question 5.
Some Suji has been stored in a rusty tin. When taken out it contains some flakes of rust. How will you remove them?
Answer:
We can remove flakes of rust by sieving the mixture.
Question 6.
What is the advantage of distillation over evaporation?
Answer:
In distillation liquid can be recovered while in evaporation it is lost.
Question 7.
Which substance is used for loading?
Answer:
Alum.
Question 8.
What is the use of alum in loading?
Answer:
Alum is used to make the decantation faster.
Question 9.
Name the process to obtain salt from sea water?
Answer:
Evaporation.
Question 10.
Name two sublime substances?
Answer:
- Iodine
- Ammonium chloride.
Question 11.
What happens during sublimation?
Answer:
Solids are directly converted into their vapours without undergoing liquid state.
Question 12.
How does doctor get distilled water?
Answer:
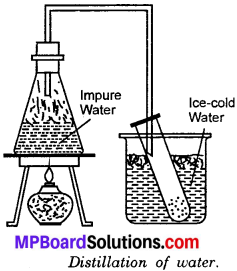
By distillation of water.
![]()
Question 13.
Can you say that a mosquito net is a filter?
Answer:
Yes, mosquito net is a filter. It filters out mosquito from the air.
Question 14.
How does jeweller separate pearls of different sizes?
Answer:
Jeweller separates pearls of different size by sieving.
Question 15.
Are all crystals of substances of same shape and colour?
Answer:
No, crystals of different substances are of different shape and colour.
Question 16.
Tea filter is missing, then what method would you use for separation of tea powder?
Answer:
Sedimentation.
Question 17.
Which organ of our body separates harmful components from blood?
Answer:
Kidney.
![]()
Question 18.
What happens when camphor is left open?
Answer:
When camphor is left open then a strong smell is produced.
Question 19.
Name two pure substances that you know?
Answer:
All elements and compounds are pure substances like salt, water, carbon (graphite), diamond, silver, copper etc.
Question 20.
Solid substance is dissolved in water. Which of the following methods is used to separate it?
- Filtration
- Evaporation
- Sublimation
- Decantation.
Answer:
A solid substance is dissolved in water. It is separated by the method of evaporation.
![]()
Question 21.
Which method would you use for the separation of a solid substance dissolved in water? Both the components are to be collected?
Answer:
Distillation is used to separate solid substance and the water from the mixture like salt solution.
Question 22.
Why does visibility increase after rains?
Answer:
This is because the dust particles that were present in air settle down due to loading by fain drops and we can see distant objects if the air dust is free and clean.
Question 23.
Name the two materials used in water filters?
Answer:
- Filters made from ceramics are used in households.
- In water – works for large supply of drinking water the material called resin, a kind of plastic is used.
Question 24.
Why is chlorine mixed with drinking water?
Answer:
In water – works, chlorine is used for killing the harmful bacteria present in water before supplied to consumers.
Question 25.
Name the method by which we can remove butter from milk?
Answer:
The process called centrifugation is used to remove butter from milk. Milk is churned in special machines like centrifuges. Butter being light in weight floats on the surface of milk and is removed easily.
![]()
Question 26.
Define evaporation?
Answer:
Evaporation method is used to separate solids dissolved in a liquid. This process is largely used to obtain common salt from sea or lake water. The process of converting a liquid into its vapour by heating is called evaporation.
Question 27.
Define crystallization?
Answer:
Crystallization method is used to purify solid substances. The process of separating a pure substance in the form of crystals from its hot saturated solution by cooling is called crystallization.
Separation of Substances Short Answer Type Questions
Question 1.
Name three mixtures commonly found in nature?
Answer:
The three mixtures commonly found in nature are soil, air and sea – water. Soil is a mixture of various inorganic and organic matter. Air is a mixture of various gases and dust particles. Sea water is a mixture of water and salts.
Question 2.
How would you separate water and mustard oil?
Answer:
The separation of water and mustard oil is based on the principle that water is heavier than oil and also the two are immixible liquids. They form distinct layers. Take the mixture in separating funnels. Separate the water by opening the stop – clock at the base of the funnel in a beaker.
Question 3.
What is the process to separate salt and camphor?
Answer:
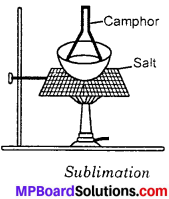
Camphor is a volatile substance. It sublimes on heating. When a mixture of salt and camphor is heated, the camphor being volatile sublimes and is collected as shown in figure. The salt being non – volatile is left behind.
![]()
Question 4.
Explain how would you make crystals of sugar?
Answer:
Prepare sugar solution in hot water. The beaker is allowed to cool. Filter the solution. Hang a crystal of sugar in this solution with the help of a thread and a glass rod or a pencil. Leave the solution uncovered and undisturbed for a few days. We observe that the crystal slowly begin to grow.
Question 5.
Write the name of methods which we use in our daily life for separating components of mixtures?
Answer:
The various processes involved in separation of a mixture components of a mixture are:
- Winnowing
- Hand – picking
- Sieving
- Decantation
- Filtration
- Magnetic separation
- Loading
- Distillation
- Centrifugation
- Crystallization
- Sublimation.
Question 6.
How you separate a mixture of milk and cooking oil?
Answer:
Milk and cooking oil are two immiscible liquids. The cooking oil is lighter than milk and it will form upper layer in this mixture. We take the mixture in a separating funnel and allow the mixture to settle. When the separation of milk and cooking oil is complete, we open the stop – cock of the separating funnel. The lower layer of milk is decanted leaving behind oil in the separating funnel. The cooking oil is collection in a separate breaker.
![]()
Question 7.
Define distillation?
Answer:
Distillation is the process of evaporation followed by condensation. This method is used to separate a mixture of two miscible liquids with a difference in their boiling points. This process is also used to obtain pure substance from a solution. In evaporation the liquid is lost but in distillation both solute and solvent are obtained. Distilled water used by the doctor for injections is obtained by distillation. In Kuwait, sea water is distilled to get drinking water.
Separation of Substances Long Answer Type Questions
Question 1.
What is a mixture? Give examples of mixtures?
Answer:
A mixture is a heterogeneous material which has two or more kind of particles. A mixture may have solid, liquid or gas components. Examples:
- Rock salt is a mixture of some salts, soil and sand.
- Soil is a mixture of clay, sand and particles of grass and other dead plants.
- Sherbet we drink in summer is a mixture of water, sugar and colouring and flavouring substances.
- Sea water is a mixture of water and many salts.
- Air is a mixture of oxygen, nitrogen, carbon dioxide, water vapour, dust particles and gases like argon.
Question 2.
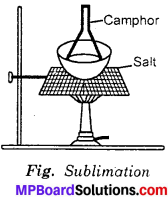
You are given a mixture of sand, water and mustard oil. How will you separate the components of the mixture?
Answer:
Principle – The separation is based on the principle that sand and mustard oil are insoluble in water. But sand being heavy, so settles at bottom and mustard oil being lighter floats on water surface. The separation is carried out in two steps.
Step – I:
Take the mixture of water, sand and mustard oil, filter the mixture. The sand will be separated. The water and oil will pass through filter – paper and will be collected as filtrate.
Step – II:
Take the filtrate in a separating funnel. Allow the mixture to set. Then open the stopcock and decant of water. Mustard oil will be left in the separating funnel.
Question 3.
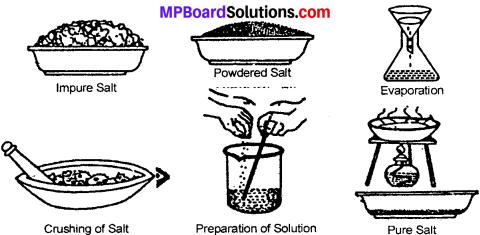
How will you obtain pure salt from its impure sample?
Answer:
Rock salt is a mixture of salt, clay and sand. Of these pure salt is soluble in water. Therefore, rock salt is first ground to a fine powder. The powdered rock salt is dissolved in water. On dissolving, salt being soluble in water so dissolves and the clay and sand is left behind:
The mixture is now filtered through a filter – paper. The clay and sand filtrate. The filtrate is evaporated in China dish. The water evaporates and pure salt is left behind.
Question 4.
How will you separate a mixture of iron filings, ammonium chloride and sand from their mixture?
Answer:
Principle – The separation is based on the principle that ammonium chloride is volatile, iron filings are magnetic and sand is neither volatile nor magnetic. The separation is carried out in two steps:
Step – I:
Take the mixture in a dish. Bring a strong bar magnet over the mixture. The iron filings are attracted to the magnet and are separated leaving the mixture of ammonium chloride and sand.
Step – II:
Take the mixture in a China dish. Cover the China dish with an. inverted funnel as shown in the fig. Heat the content of China dish with spirit lamp. The ammonium chloride being volatile sublimes and its vapours collects on the surface of funnel. Allow the funnel to cool. Scratch the ammonium chloride. The sand will remain in China Dish.
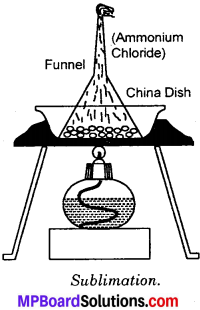
Question 5.
Define threshing with diagram?
Answer:
The process that is used to separate grain from stalks is threshing. In this process, the stalks are beaten to free the grain seeds (Fig.). Sometimes, threshing is done with the help of bullocks. Machines are also used to thresh large quantities of grain.

Question 6.
You know various methods of separating mixtures. Here four pictures are shown. Write the correct names of methods under each picture?
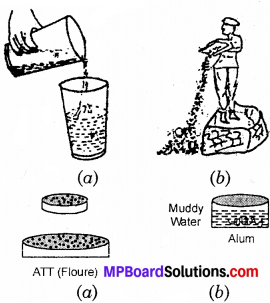
Answer:
- Decantation.
- Winnpwing
- Sieving
- Loading.
Question 7.
How will you separate pure water from a solution of salt in water?
Answer:
Take the mixture of salt in water in a flask. Set the arrangement for distillation. On heating water vapour rises and passes through the tube. They are condensed in the test – tube surrounded by the cold water. Thus we get pure water leaving behind salt in the flask.