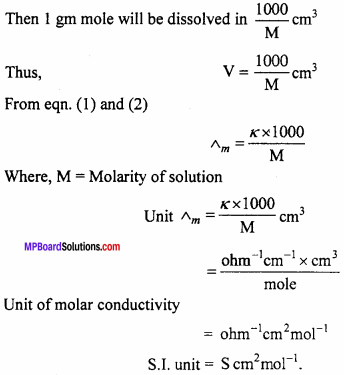
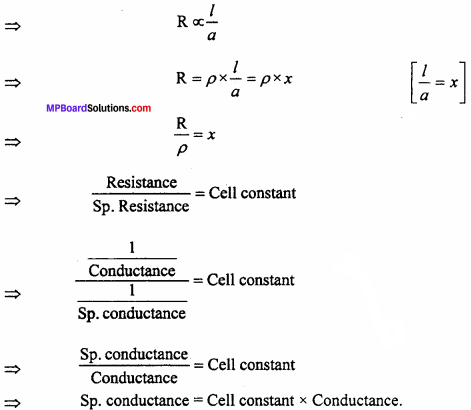
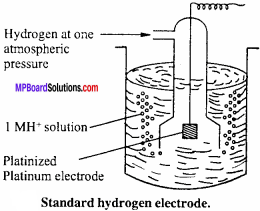
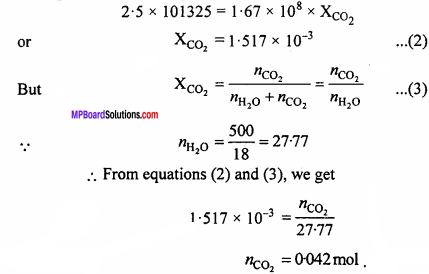
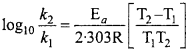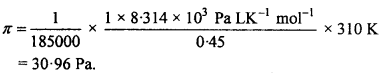MP Board Class 12th Chemistry Important Questions Chapter 7 The p-Block Elements
The p-Block Elements Important Questions
The p-Block Elements Objective Type Questions
Question 1.
(A) Choose the correct answer :
Question 1.
In which compound oxygen exhibits +2 oxidation state :
(a) H2O
(b) Na2O
(c) OF2
(d) MgO.
Answer:
(c) OF2
Question 2.
Reddish brown gas is formed, when nitric oxide oxidizes by air. This gas is :
(a) Na2O2
(b) Na2O4
(c) NO2
(d) N2O3.
Answer:
(c) NO2
Question 3.
H3PO3 is :
(a) Dibasic acid
(b) Monobasic acid
(c) Tribasic acid
(d) Tetrabasic acid.
Answer:
(c) Tribasic acid
Question 4.
Oxide which shows paramagnetic character :
(a) N2O4
(b) NO4
(C) P4O6
(d) N2O5
Answer:
(b) NO4
Question 5.
Ammonia can be dried by :
(a) H2SO4
(b) P2O5
(c) Anhydrous CaCl2
(d) CaO.
Answer:
(d) CaO.
Question 6.
Nitric acid change iodine into :
(a) Iodic acid
(b) Hydroiodic acid
(c) Iodine pentaoxide
(d) Iodine nitrate.
Answer:
(a) Iodic acid
![]()
Question 7.
NH3 is a Lewis base which forms complex salt with cations. Following cation does not form complex salt with NH3 :
(a) Ag+
(b) Cu2+
(c) Cd2+
(d) Pb2+.
Answer:
(d) Pb2+.
Question 8.
Ammonia is soluble in :
(a) Hg2Cl2
(b) PbCl2
(c) Agl
(d) Cu(OH)2.
Answer:
(d) Cu(OH)2.
Question 9.
Bleaching action of SO2 is due to :
(a) Reduction
(b) Oxidation
(c) Hydrolysis
(d) Acidic nature.
Answer:
(a) Reduction
Question 10.
What happens when SO2 is passed through acidic K2Cr2O7 :
(a) Solution turns blue
(b) Solution turns colourless
(c) SO2 reduced
(d) Green chromic sulphate is formed.
Answer:
(d) Green chromic sulphate is formed.
Question 11.
P2O2 forms which of the following acid :
(a) H4P2O7
(b) H3PO4
(c) H3PO3
(d) HPO3.
Answer:
(c) H3PO3
Question 12.
Most acidic halide is :
(a) PCl5
(b) SbCl3
(c) BrCl3
(d) CCl4
Answer:
(a) PCl5
Question 13.
The catalyst used in manufacturing of H2SO4 is :
(a) Al2O3
(b) CrO3
(c) V2O5
(d) MnO2
Answer:
(c) V2O5
Question 14.
The hydrolysis of phosphorus triha lides give:
(a) One monobasjc acid and one dibasic acid
(b) One monobasjc acid and one tribasic acid
(c) One monobasjc acid and a salt
(d) Two dibasic acids.
Answer:
(a) One monobasjc acid and one dibasic acid
Question 15.
In the following reaction:
P4 + 3NaOH + 3H2O → PH3 + 3NaH2 PO2
(a) P oxidises
(b) P oxidises and reduces
(c) P reduces
(d) Na oxidises.
Answer:
(b) P oxidises and reduces
Question 16.
Laughing gas is:
(a) NO
(b) N2O
(c) N2O3
(d) N2O5.
Answer:
(b) N2O
Question 17.
White phosphorus does not contain:
(a) 6 P – P single bond
(b) 4 P – p single bond
(c) 4 lone pair of electron
(d) Bond angle of P – P – P is 600.
Answer:
(b) 4 P – p single bond
![]()
Question 18.
On heating NH4CI and NaNO2 solution gives :
(a) N2O
(b) N2
(c) NO2
(d) NH3.
Answer:
(b) N2
Question 19.
Formula of metaphosphoric acid is:
(a) H3PO3
(b) HPO3
(c) H3PO3
(d) H2PO3.
Answer:
(b) HPO3
Question 20.
Gas which cannot be collected on water:
(a) Na
(b) O2
(c) SO3
(d) PH3.
Answer:
(c) SO3
(B) Choose the correct answer:
Question 1.
Chlorine shows bleaching property in the presence of :
(a) Dry air
(b) Moisture
(c) Sunlight
(d) Pure oxygen.
Answer:
(b) Moisture
Question 2.
Which of the following element forms least number of compounds :
(a) He
(b) Ar
(c) Kr
(d) Xe.
Answer:
(a) He
Question 3.
Which is used for bright advertisement display :
(a) Xe
(b) Ar
(c) Ne
(d) He.
Answer:
(c) Ne
Question 4.
Monazite is a source of:
(a) Ne
(b) Ar
(c) Kr
(d) He.
Answer:
(d) He.
![]()
Question 5.
Which of the following is not obtained by the direct reactions of the constituents :
(a) XeF2
(b) XeF4
(c) XeO3
(d) XeF6.
Answer:
(d) PbI4.
Question 6.
Which of the following halides are least stable and whose existence is suspicious :
(a) CI4
(b) Gel2
(c) Snl4
(d) PbI4.
Answer:
(d) PbI4.
Question 7.
Which of the following is the strongest acid :
(a) HBr
(b) HCl
(c) HF
(d) HI.
Answer:
(d) HI.
Question 8.
Most electronegative element is :
(a) F
(b) Cl
(c) Br
(d) I.
Answer:
(a) F
Question 9.
Which halogen is solid at room temperature :
(a) Cl2
(b) I2
(c) Br2
(d) F2.
Answer:
(d) F2.
![]()
Question 10.
Which has maximum electron affinity :
(a) F
(b) Cl
(c) Br
(d) I.
Answer:
(b) Cl
Question 11.
Electronic configuration of valence shell of halogen is :
(a) s2p5
(b) s2p3
(c) s2p6
(d) s2p4.
Answer:
(a) s2p5
Question 12.
Which element is most alkaline:
(a) F
(b) Cl
(c) Br
(d) I.
Answer:
(d) I.
Question 13.
The strongest reducing agent is:
(a) F–
(b) Br–
(c) I–
(d) Cl–
Answer:
(c) I–
Question 14.
Which of the following is weakest acid:
(a) HF
(b) HCl
(c) HBr
(d) HI.
Answer:
(a) HF
Question 15.
Oxidizing property is highest of:
(a) 12
(b) Br2
(c) F2
(d) Cl2.
Answer:
(c) F2
![]()
Question 16.
Which Noble gas does not have octet complete:
(a) Helium
(b) Neon
(c) Argon
(d) Krypton.
Answer:
(a) Helium
Question 17.
Which Halogen sublimates :
(a) Chlorine
(b) Bromine
(c) Iodine
(d) Fluorine.
Answer:
Question 18.
12 easily dissolve in Kl solution to form :
(a) I
(b) KI2
(c) KI
(d) KI3
Answer:
(d) KI3
Question 19.
Gas mixed in oxygen which is used in respiration for asthma patients :
(a) N2
(b) Cl2
(c) He
(d) Ne.
Answer:
(c) He
Question 20.
Deacon’s process is used for manufacture of :
(a) Bleaching powder
(b) Chlorine
(c) Nitric acid
(d) Sulphuric acid.
Answer:
(b) Chlorine
Question 21.
Sea grass is the source of industrial manufacture of :
(a) Chlorine
(b) Bromine
(c) Iodine
(d) Fluorine.
Answer:
(c) Iodine
Question 2.
(A) Fill in the blanks :
- N2O is a ……………….. oxide.
- Formula of Caro’s acid is ………………..
- Concentrated nitric acid has brown colour due to the dissolution of ……………….. gas.
- Out of nitrogen oxides ……………….. and ……………….. are paramagnetic.
- Pyrophosphoric acid is ……………….. basic acid.
- H2S gas cannot be dried by cone. H2SO4 because H2S ……………….. it.
- Fuming sulphuric acid dissolves in SO3 to form ………………..
- Oxidation state of S in H2S2O8 (Marshall gas) is ………………..
- NH3 gives white fumes of ……………….. when it combines with HCl.
- Elements of 16th group are known as ………………..
- ……………….. is used as a refrigerant.
Answer:
- Neutral
- H2SO5
- NO2
- NO
- NO2
- Tetra
- Reduces
- Oleum
- +6
- NH4Cl
- Chalcogen
- Liquid NH3.
![]()
(B) Fill in the blanks :
- …………………. has the highest electron affinity.
- In the presence of moisture, chlorine acts as a ………………… agent.
- Bleaching powder is also called as …………………
- At ordinaiy temperature bromine is a …………………
- Inter halogen compound AX5 has ………………… structure.
- Chlorine was discovered by …………………
- Neil Bertlett forms first noble compound which is …………………
- Element with highest electron affinity is …………………
- In oxy acid halogen present in ………………… hybride state.
- Gas which is light due to which it is filled in aircraft tyres is …………………
- ………………… inert gas is mostly used in advertisements.
- Elements of Group 17th are commonly known as …………………
- ………………… is a radioactive inert gas.
Answer:
- Chlorine
- Bleaching
- Calcium chlorohypochloride
- Liquid
- Square pyramidal
- Scheele
- Xe [PtF6]
- Chlorine,
- sp3
- Helium
- Ne (Neon)
- Halogen
- Radon.
Question 3.
(A) Answer in one word / sentence :
- Who protects earth from ultraviolet rays?
- Give the chemical name of oil of vitriol or king of chemical.
- Which gas is used for refrigeration?
- At which temperature water has maximum density?
- Give the name of compound formed by the dissolution of S03 in sulphuric acid.
- Write name of an antichlor.
- Which gas is used for bleaching of oil and elephant teeth?
- Ammonium salts react with Nessler’s reagent to give which coloured precipitate?
- On moving down from N to Bi, +3 oxidation state of Bi becomes more stable than +5. Why?
- State the percentage of N2 by volume in nature.
Answer:
- Ozone layer
- H2SO4
- NH3
- 4°C
- Oleum
- SO3
- Ozone
- Brown
- Due to Inert pair effect
- 80%.
(B) Answer in one word / sentence :
- Give the name of radioactive halogen.
- Which noble gas is used in bulb with nitrogen? (MP 2018)
- Write the name of noble gas which is used in treatment of cancer.
- Write the formula of Camalite.
- Which noble gas is maximum available in the atmosphere?
- What is the shape of XeF6?
- Write one use of fluorine.
- Which type of hybridization is present in XeO3?
- Write the oxidation state of F.
- Write only equation for the preparation of chlorine in the lab.
- What is the shape of AX3 type of Interhalogen compound?
- Write the correct order of strength of halogen acid.
- Which noble gases do not form compounds?
- With which noble gas does F form compounds?
- Sea weeds are the source of which halogen?
Answer:
- Astatine
- Ar
- Rn
- KCl.MgCl2.6H2O
- Argon
- Distorted octahedron
- In preparing fluorocarbon, which is used in refrigeration
- sp3
- -1
- MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
- T – shape
- HF < HCl < HBr < HI
- He, Ne and Ar
- Xe
- Iodine.
Question 4.
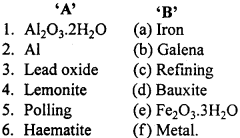
Match the following :
I.

Answer:
- (d)
- (c)
- (a)
- (b)
- (f)
- (g)
- (e)
- (i)
- (j)
- (h).
II.

Answer:
- (e)
- (c)
- (b)
- (a)
- (d)
III.

Answer:
- (e)
- (d)
- (b)
- (f)
- (c)
- (a)
General Principles and Processes of Isolation of Elements Very Short Answer Type Questions
Question 1.
What are clathrate compounds?
Answer:
In the cavity or space of crystal lattice of a compound small sized elements like noble gases get trapped within. Such compounds are called clathrate compounds.
Example : Kr3 (β – quinol).
Question 2.
Why is BiH3 the strongest reducing agent amongst all the hydrides of Group 15 elements? (NCERT)
Answer:
Among the hydrides of Group – 15, BiH3 is the least stable because Bi has largest size in group and has least tendency to form covalent bond with small hydrogen atom. Therefore, it can readily lost H atom and has strongest tendency to act as reducing agent.
Question 3.
Why is N2 less reactive at room temperature? (NCERT)
Answer:
In N2 molecule, there is a strong pπ – pπ overlap that results in the formation of triple bond between N atoms (N ≡ N). As a consequence, the bond dissociation energy of N2 is very high and N2 is less reactive at room temperature.
![]()
Question 4.
Why is Helium used in diving apparatus? (NCERT)
Answer:
Helium is used as a diluent for oxygen is modem diving apparatus because of its very low solubility in blood.
Question 5.
Noble gases are inert. Why?
Answer:
Noble gases are inert due to following reasons :
Noble gases are inert because octet of these gases are complete. It is the most stable state and do not have unpaired electrons. Ionisation energy of noble gases are high and electronegativity and electron affinity are zero. Therefore, these gases neither accept electron nor release electron. Thus, noble gases are inert in nature.
Question 6.
Complete the following reactions: (NCERT)
(i) C2H2 + O2 →
(ii) 4AI + 3O2 →
Answer:

Question 7.
Arrange the following in the order of property indicated for each set: (NCERT)
(i) F2, Cl2, Br2,I2 – increasing bond dissociation enthalpy.
(ii) HF, HCl, HBr, HI – increasing acid strength.
(iii) NH3, PH3, ASH3, SbH3, BiH3 – increasing base strength.
Answer:
(i) I2 < F2 < Br2 < Cl2
![]()
Here F2 has very less bond dissociation energy inspite of having small size. It is due to repulsion between the lone pair of electrons on small size F – atoms.
(ii) HF < HCl < HBr < HI
It depends upon their bond dissociation enthalpy, which decreases from H – F to H – I as the size of atom increases from F to I.
(iii) BiH3 < SbH3 < AsH3 < PH3 < NH3.
They are Lewis bases due to presence of lone pair of electrons on central atom of hydrides. The availability of lone pair of electrons is maximum in nitrogen owing to its small size therefore, NH3 is maximum basic and as the size of central atom increases availability decreases.
Question 8.
Describe the laboratory preparation of chlorine.
Answer:
Laboratory preparation:
HCl and MnO2 is taken in a round bottom flask. Cone. HCl is added from thistle funnel. The flask is heated slowly so that green yellow colour of Cl2 gas evolved.
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Question 9.
Fluorine is strong oxidizing agent as compared to chlorine. Why?
Answer:
Because of following points, fluorine is strong oxidizing agent:
- The size of F atom is smaller than Cl atom.
- Electronegativity of fluorine is higher than Cl atom.
- Dissociation energy of fluorine is less than that of chlorine.
- E° value of fluorine is more than chlorine.
Question 10.
F2O is not considered to be the oxide of fluorine. Why?
Answer:
Fluorine is the most electronegative element. Its electronegativity is higher than oxygen. In naming a compound less electronegative elements are first and then more electronegative elements are written, Therefore, F2O or OF2 is known as oxygen difluoride and not fluorine oxide.
![]()
Question 11.
Interhalogen compounds are more reactive than halogen. Why?
Answer:
X – Y bonds of interhalogen compounds are more polar due to difference in the electronegativities of halogen and are generally weaker than X – Y bonds of pure halogen. Therefore, interhalogen compounds are more reactive than halogens.
Question 12.
Helium and Neon do not form compounds with Fluorine. Why?
Answer:
Due to absence of d – orbitals in the valence shell of He and Ne, their electrons cannot get excited into higher energy d – sub – shells. Therefore, He and Ne do not form compounds with fluorine.
Question 13.
Explain why NH3 is basic while BiH3 is only feebly basic. (NCERT)
Answer:
Atomic size of N(70pm) is much smaller than that of Bi (148 pm). Therefore, electron density on the N – atom is much higher than that on Bi – atom. Consequently, the tendency of N in NH3 to donate the pair of electrons is much higher than that of Bi in BiH3. Thus, NH3 is basic while BiH3 is only feebly basic.
The p – Block Elements Short Answer Type Questions
Question 1.
Explain with reason :
- HF is liquid while other hydrides of halogens are gases at normal temperature.
- Fluorine does not form polyhalide.
Answer:
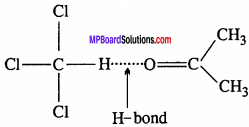
1. The electronegativity of fluorine is maximum than other halogens. Therefore, HF molecule is conjugated by H – bond and the boiling point of HF is more to other halogen acids therefore HF is liquid while halides of other halogens are gases at room temperature.
H – z….H – F….H – F….H – F
2. The electronic configuration of fluorine is 1s2, 2s2, 2p5. Due to absence of orbital in its valency shell it does not show higher oxidation state and so it does not made polyhalide.
Question 2.
How is SO2 an air pollutant? (NCERT)
Answer:
SO2 dissolves in rain water and produces acid rain. The acid rain contains sulphuric acid.
![]()
In addition to H2SO4, acid rain also contains HNO3.
![]()
Question 3.
Why are halogens strong oxidising agents? (MP 2018, NCERT)
Answer:
Halogens are strong oxidising agents due to their low bond dissociation enthalpy, high electronegativity and large negative electron gain enthalpy. Halogens have a strong tendency to accept electrons and thus get reduced.
X2 + 2e– → 2X–
As a result, halogen acts as strong oxidising agents. Their oxidising power however decreases from F2 to I2 as is evident from their electrode potentials :
E°F2/F– = + 2.87 V
E°Cl2/Cl– = + 1.09 V
E°Br2/Br = +1.09 V
E°Br2/I = + 0.54 V
Question 4.
Why does the reactivity of nitrogen differ from phosphorus? (NCERT)
Answer:
Molecular nitrogen exist as a diatomic molecule having a triple bond between the two nitrogen atoms, N ≡ N (due to it stability to form pπ – pπ multiple bonding). The bond dissociation energy is very high (941.4 kJ mol-1). Thus, under ordinary conditions, nitrogen behaves as an inert gas. On the other hand, white and yellow phosphorus exists as a triatomic molecule (P4) having single bonds. The dissociation energy of P – P bond is low (213 kJ mol-1). Thus, phosphorus is much more reactive than nitrogen.
Question 5.
Give the reason for bleaching action of Cl2. (NCERT)
Answer:
Bleaching action of chlorine is due to its oxidation. In the presence of moisture, chlorine gives nascent oxygen.
Cl2 + H2O → 2HCl + [O]
Because of nascent oxygen, it bleaches colouring substance as
Colouring substance + [O] → Colourless substance.
It bleaches vegetables or organic matter. The bleaching action of chlorine is permanent.
Question 6.
Explain, why:
- Noble gases are monoatomic?
- Atomic radii of noble gases are largest?
- Ionisation energy of noble gases are highest?
Answer:
- There is no any unpaired electron in electronic configuration of noble gases. So these do not form chemical bonds and are monoatomic.
- Outermost shell of noble gases are completely filled so atomic radii of noble gases are maximum.
- All electrons in outermost orbit of noble gases are paired and energy required to make them unpaired is very high. Thus, noble gases have maximum ionisation energy in respective periods.
Question 7.
Why does nitrogen show catenation properties less than phosphorus? (NCERT)
Answer:
The catenation properties depends upon the strength of the element – element bond. The N – N bond strength is much weaker (due to repulsion of lone pairs on nitrogen because of its small size) than the P – P bond strength, therefore, nitrogen shows catenation less than phosphorus.
![]()
Question 8.
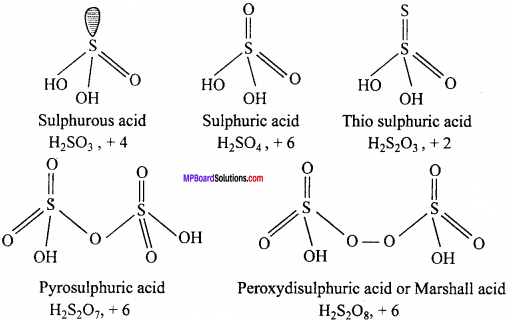
Write the formulae and structure of any five oxy – acids of sulphur.
Answer:
Main oxyacids of sulphur and oxidation state of sulphur in them are as follows :
Question 9.
Contact process of manufacturing of H2SO4 is supposed to be more advance than lead chamber process. Why?
Answer:
- The acid obtained by contact process is pure while the acid obtained by lead chamber process is impure.
- The plant for lead chamber process requires large area while plant used in contact process occupies comparatively less area.
- The catalyst used in contact process is platinised asbestos while in lead chamber process catalyst is gaseous (NO2 gas) and to maintain its flow regular is tedious.
- In contact process concentrated sulphuric acid is obtained while in lead chamber process dilute acid is obtained.
- In contact process running of the plant is less costly while in lead chamber process running of the plant is costly.
Question 10.
Why does NH3 form hydrogen bond but PH3 does not? (NCERT)
Answer:
The N – H bond in ammonia is quite polar as nitrogen is highly electronegative in nature. As a result, NH3 molecules are linked by intermolecular hydrogen bonding.
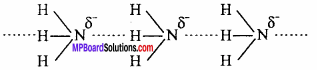
On the other hand, P – H bond is non – polar as both P and H have same electronegativity. Hence in phosphine no hydrogen bonding is present.
Question 11.
Bond angle in PH4+ is higher than that in PH3. Why? (NCERT)
Answer:
PH4+ is a regular tetrahedral structure, thus bond angle is 109°28′.
PH3 molecule has a pyramidal structure. The bond angle is 93°. Hence, bond angle in PH4+ ion is higher.
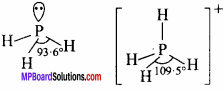
Question 12.
What happens when white phosphorus is heated with concentrated NaOH solution in an inert atmosphere of CO2? (NCERT)
Answer:
When white phosphorus is heated with cone. NaOH solution in an inert atmosphere of CO2, phosphine gas is prepared.
![]()
Question 13.
What happens when PCl5 is heated? (NCERT)
Answer:
PCl5 has three equatorial and two axial bonds. Since axial bonds are weaker than equatorial bonds, therefore, when PCl5 is heated, the less stable axial bond breaks to form PCl3.
![]()
When heated, it sublimes but decomposes on stronger heated.
Question 14.
How are Xenon fluorides XeF2, XeF2 and XeF6 prepared?
Answer:
Xenon Fluorides:
Three fluorides of xenon are important. These are xenon difluoride (XeF2), xenon tetrafluoride (XeF4) and xenon hexafluoride (XeF6).The fluorides of xenon can be prepared by the direct combination between xenon and fluorine under different conditions.
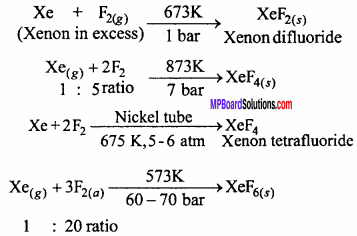
Question 15.
Write the difference between the bleaching action of SO2 and Cl2.
Or,
Bleaching of flowers by Cl2 is permanent while bleaching by SO2 is temporary, why?
Answer:
Bleaching action of SO2:
Bleaching by SO2 is due to the process of reduction in the presence of moisture.
SO2 + 2H2O →H2SO4 + 2 [H] (nascent)
The nascent hydrogen liberated in the reaction is responsible for bleaching flower but when the flower comes in contact with air. It gets oxidized and become coloured.
Coloured substance + [H] → Colourless \(\underrightarrow { air }\)Colour
Bleaching action of Cl2:
Bleaching by Cl2 is due to the process of oxidation in the presence of moisture.
Cl2 + H2O → 2HCl + [O ] (nascent)
Coloured substance + [O] > Colourless
The oxygen liberated in the reaction is responsible for bleaching, colour bleached by Cl2 is permanent and original colour cannot be restored on exposure to air.
![]()
Question 16.
Write main differences between the properties of white phosphorus and red pljosfrtiorus. (NCERT)
Answer:
Comparison of properties of White and Red phosphorus :
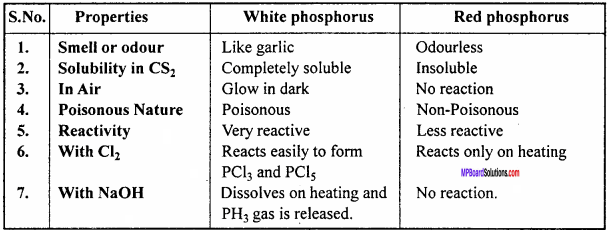
Question 17.
Give equations for the reaction of chlorine with the following :
- NH3
- NaOH
- H2O
- Bleaching property.
Answer:
Reaction of chlorine :
1. With NH3 :
(a) Cl2 reacts with excess of NH3 and form NH4Cl and N2.
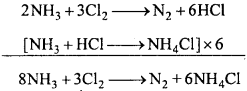
(b) When chlorine is taken in excess, an explosive product nitrogen trichloride is formed.
NH3 +3C12 → NC13 +3HCl
2. With NaOH :
(a) Cold and dilute NaOH reacts with Cl2 and form chloride and hypochlorite.
Cl2 + 2NaOH → NaCl + NaClO + H2O
(b) Hot and concentrated caustic soda reacts with Cl2 and form chloride and chlorate.
3Cl2 + 6NaOH → NaC1O3 + 5NaCl + 3H2O
3. With H2O :
Cl2 reacts with water and form chlorine water which is called hypochlorous acid which decomposes into HCl.
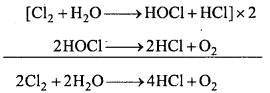
4. Bleaching action:
In presence of moisture chlorine bleaches coloured object and vegetables. It decolourises the coloured substance. This property is due to oxidation.

Thus, bleaching by chlorine is a permanent process.
![]()
Question 18.
Give reason :
- Iodine exhibits some metallic property.
- Halogens are strongly oxidizing, Why? (MP 2018)
Answer:
1. Ionization energy of iodine is small so it gives out one electron from its valence shell. In some reaction Iodine gives I+ ion so it has some metallic property.
I → I+ + e–
2. Halogens are only one electron short to complete their octet and attaining stable structure. So halogens have strong tendency to accept electron. Thus, halogens are strong oxidising agents because of accepting electrons in reduction, oxidising power of halogens decreases from fluorine to iodine.
F2 > Cl2 > Br2 > I2
The p-Block Elements Long Answer Type Questions
Question 1.
What are interhalogen compounds? How many types are they? Give one example of each type with structure.
Or,
Explain hybridization in ABS and AB? type of interhalogen compounds and draw their structures.
Answer:
Because of difference in the electronegativity value of halogen member it is possible to combine to form compound. When two different halogen combine together to form binary compound, then it is known as interhalogen compound. There are four types of interhalogen compound, which has general formula XYn, where n = 1, 3, 5 or 7.
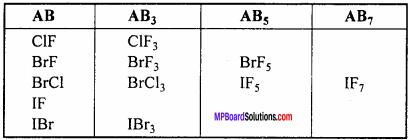
Structure of interhalogen compound:
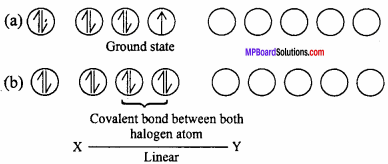
1. AB Type :
Like ClF, BrCl, IBr, ICl. Their geometry is linear. In the ground state electronic configuration of chlorine it is clearly observed that there is one unpaired electron which forms covalent bond with other halogen atom.
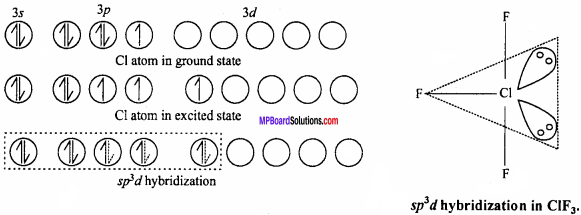
2. AB3 Type :
It is T – shaped. Like ClF3molecule, Its central atom X shows sp3 hybridization.
3. AB5 (IF5 ,BrF5 )TyPe :
These type of compounds show sp3 d2 hybridization. Then structure is square pyramidal in which there is lone pair of electron at one place. Like in the figure formation of IF5 molecule is shown.
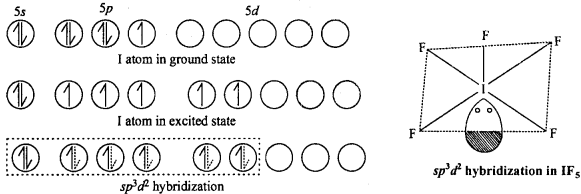
4. AB7(IF7) Type :
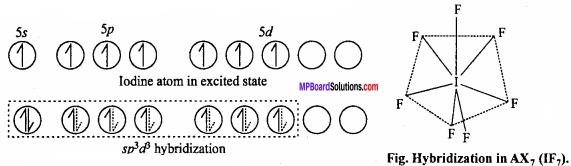
Its geometry is pentagonal bipyramidal which is formed by sp3d2 hybridization. Hybridization in IF7 molecule is sp3d3.
Question 2.
Write the uses of Noble gases.
Answer:
Uses of Noble Gases :
Uses of Helium :
- It is used in filling air ships and balloons for weather study as it is light and non – inflammable.
- Helium is used in research work for maintaining very low temperatures.
- It is used in producing inert atmosphere, for food preservation and as filler in electric transformer.
- Sea divers use mixture of helium and oxygen for deep sea diving.
This is due to the fact that under high pressure helium is less soluble in blood as compared to nitrogen. If for breathing in deep sea diving air is used then more nitrogen will dissolve in blood under pressure and when the diver comes back at the surface of the sea, the solubility of nitrogen in blood decreases due to decrease in pressure and changes into nitrogen bubbles. This causes severe pain in diver’s body. It may also cause blisters and vomiting.
Uses of Neon:
Neon lights are used for commercial advertisements. It consists of a long tube fitted with electrodes at both ends. On filling the tube with neon gas and passing electric discharge of about 1000 volt potential, a bright red light is produced. Different colours can be obtained by mixing neon with other gases. For producing blue or green light neon is mixed with mercury vapours.
Uses of Argon:
- Like helium, it is also used to create inert atmosphere in welding of aluminium and stainless steel.
- It is filled in electric bulbs along with 25% nitrogen.
- It is also used in radio valves.
- Argon alone or its mixture with neon is used in tubes for producing lights of different colours.
Uses of Krypton and Xenon:
- They are also used in electric bulbs in place of argon, but they are costly.
- Both are used in flash photography.
- Krypton is mainly used in producing fluorescence, incandescence and electric lights.
- Krypton lamp is used as indicator at aeroplane terminals for landing of the aeroplane.
Uses of Radon:
- In treatment of malignant growths (cancer) and non – healing wounds. Also, used in researches associated with radioactivity.
- Used as a substitute for X – rays in radiology.
- Radon is soluble in fatty compounds and recently a radon containing ointment has been manufactured for radiotherapy.
![]()
Question 3.
Explain manufacture of chlorine under the following heads :
Answer:
- Labelled diagram of Nelson cell
- Principle
- Deacon’s process.
Answer:
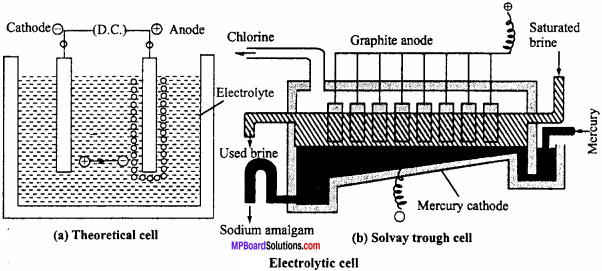
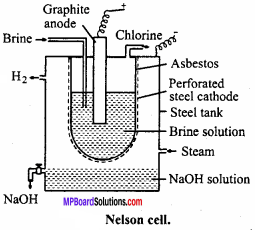
1. Nelson cell:
In Nelson cell, graphite anode is suspended in a perforated cylindrical steel cathode provided with asbestos. Brine solution is taken in steel cathode. On electrolysis, chlorine gas is liberated at anode and is drawn off through the outlet at the top. Na reacts with H2O at bottom to form NaOH and H2. Hydrogen escapes through the exit.
2. Principle:
Nelson method or By electrolysis:
Chlorine is manufactured by electrolysis of NaCl solution, chlorine is obtained as by product.
NaCl ⇌ Na+ + Cl–
At cathode : 2Na+ + 2e– → 2Na
2Na+ + 2H2O → 2NaOH + H2
At anode : cl+ + e– → Cl
Cl + Cl → Cl2
Deacon’s process:
Previously chlorine was manufactured by Deacon’s process. In this process, HCl gas is heated with oxygen at 450°C in presence of CuCl2 catalyst.
![]()
Question 4.
Explain Siemen – Halskey ozonizer processor the manufacture of ozone and draw labelled diagram.
Answer:
For commercial production of ozone Siemen – Halskey ozonizer is used. It consists of an iron box fitted with 6 to 8 glass cylinders. Each cylinder is 3 ft high and 10″ in width. The box is divided into three compartments. Cold water flows through the central compartment for cooling. Each cylinder is fitted with an aluminium rod which are resting on insulating glass slab in the lower compartment.
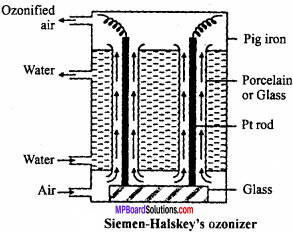
There is annular space between rod and sides of cylinder. A current of air flows through this annular space. The aluminium rods are subjected to high potential of 8,000 to 10,000 volts. The air which enters at bottom escapes at the top and contains about 5% of ozone. If pure oxygen is used in place of oxygen then ozonized oxygen contains about 15% of ozone.
Question 5.
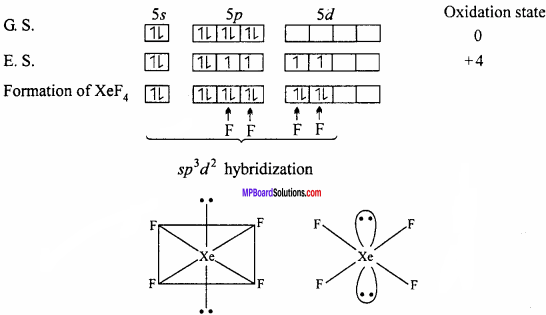
Explain the structures of XeF2 and XeF4.
Answer;
Structure of XeF2:
In XeF2 molecule Xe atom is in sp3d hybrid state hence, its structure is trigonal bipyramidal but its geometry is linear.
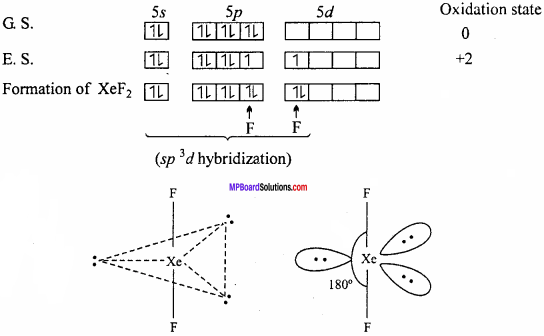
Structures of XeF4:
XeF4 formed under second excited state of Xe where two of sp – orbitals are unpaired and 4 unpaired electron develop. Being sp3d2 hybridization involved, the structure is octahedral but due to presence of two lone pair of electron geometry molecules get distorted and becomes square planar.
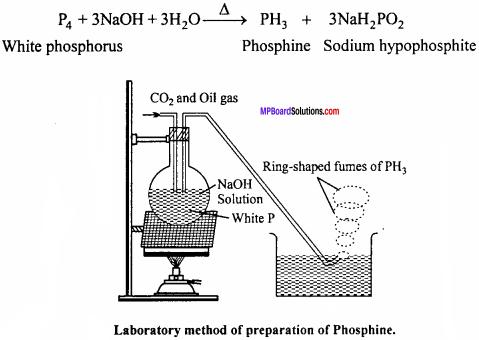
Question 6.
Dtim labelled diagram of laboratory method of phosphine and give chemical emurfion.
Answer:
Phosphine is prepared in laboratory by heating white phosphorus with NaOH in an inert atmosphere of CO2 and oil gas. Phosphine produced is highly inflammable due to the presence of phosphorus dehydride as impurity. Vapour of phosphine form vortex noing of smoke in contact with air. Alcoholic KOH can be used in place of NaOH.
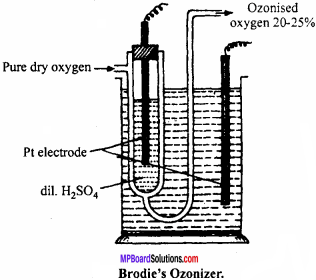
Question 7.
Describe Brodie’s Ozonizer with diagram.
Answer:
It is U – shaped glass tube, consists Pt wire with dil. H2SO4. The whole apparatus is put into glass utensil, outer utensil also contain H2SO4 and Pt electrode. Dry O2 is passed through U – tube under the influence of high electric discharge oxygen gets converted to ozonised oxygen, the outcoming gas contains 20% of ozone.
Question 8.
Give chemical reaction of ozone with
- K2MnO4
- I2
- Ag2O
- CH2 ≡ CH2 and (v) PbS.
Answer:
1. With K2MnO4 : Potassium permangnate is formed.
2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2
2. With I2 : Iodic acid is formed.
I2 + H2O + 503 → 2HIO3 + 5O2
3. With Ag2O : Silver is formed.
Ag2O + O3 → 2Ag + 2O2
4. With CH2 = CH2 : Ethene ozonide is formed.
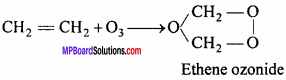
5. With PbS : PbS04 is formed.
PbS + 4O3 → PbSO4 + 4O2
Question 9.
Explain Ostwald’s process of manufacture of nitric acid drawing labelled diagram.
Answer:
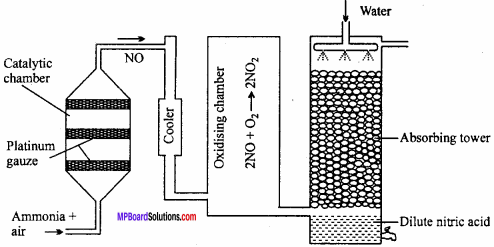
Principle of Ostwald’s process:
The mixture of ammonia and air when passed over platinum gauze catalyst at 750 – 900°C, ammonia gets oxidized to nitric oxide.
![]()
The reaction is exothermic and the heat liberated maintains the temperature of the catalyst. The nitric oxide is then oxidised to nitrogen dioxide by air which is cooled to 50°C and absorbed by water to produce nitric acid.
Question 10.
Describe the manufacture of H2SO4 by contact process. (NCERT)
Answer:
Manufacture of sulphuric acid:
Sulphuric acid is prepared on large scale by contact process. The basic raw material used is either sulphur or iron pyrites.
Principle of contact process:
When pure and dry SO2 mixed with air is passed over V2O5 catalyst it gets oxidised to SO3 which is absorbed by H2SO4 to form oleum i.e,, H2S2O7.
2SO2 + O2 → 2SO3 + 45.2 cal
SO3 + H2SO4 → H2S2O7
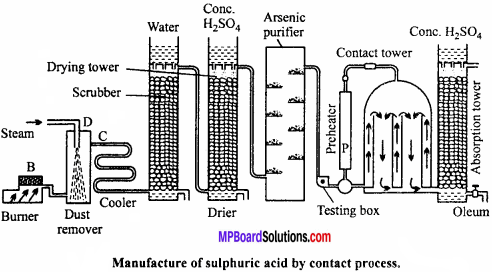
Details of the process are given below :
(a) Sulphur or pyrite burners : SO2 is produced by burning sulphur or roasting pyrites in pyrite burners.
(b) Purification unit: This unit is an assembly of various parts like.
(i) Dust chamber : SO2 gas produced is passed through dust chamber through which steam is sprayed from top. Dust particles absorb moisture, become heavy and settle down.
(ii) Cooling pipes : Now, the gaseous mixture is passed through cooling pipes to lower the temperature to 100°C.
(iii) Washing tower or scrubbers : It is filled with quartz and showers of cold water continue from top. Dust particles as well as soluble impurities settle down and are removed.
(iv) Drying tower : It is a high tower full of quartz pieces. Cone. H2SO4 is sprayed from the top. Gases enter the tower from bottom and get dried in contact with cone. H2SO2.
(v) Arsenic purifier : It is filled with shelves containing freshly prepared ferric hydroxide which absorb the impurities of arsenic purified and dried.
(c) Testing box:
Before sending the gaseous mixture to contact chamber it is tested here. A strong beam of light is thrown into the testing box, if the gaseous mixture contains dust or any other particles they become visible, hence gaseous mixture is repurified. The completely pure gas is then sent to the contact chamber.
(d) Preheater:
Pure gas free from dust particle is heated in preheater to 450°C and then passed into contact tower, where SO2 gets oxidized to SO3. This reaction is exothermic due to which temperature of contact tower raises to 450°C. After this the pure gases are pumped directly into the contact tower.
(e) Contact tower:
It consists of a big iron container containing several pipes filled with vanadium pentoxide or platinized asbestos or any other suitable catalyst. Temperature is maintained at 450°C. Pure and dry SO2 reacts with air in these pipes to form sulphur trioxide.
![]()
On account of exothermic nature of reaction, sufficient heat is available.
(f) Absorption tower:
The sulphur trioxide is then passed into a tower in which cone, sulphuric acid flows down in the form of spray. Sulphuric acid absorbs SO3 and becomes more concentrated. This acid, due to excess of SO3 produces a fog in the tower. The acid obtained is called fuming sulphuric acid or oleum.
![]()
Oleum is mixed with calculated quantity of water to form acid of a particular strength.
H2S2O7 + H2O → 2H2SO4
It may be noted that sulphur trioxide is not directly absorbed in water to form sulphuric acid because it forms a dense fog of sulphuric acid which does not condense easily.
![]()
Question 11.
Write formula, structure and oxidation state of any three oxy – acids of chlorine
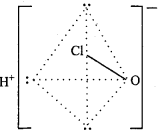
Answer:

Structure:
Structure of HClO:
The central atom Cl in CIO ion of HCIO, forms four hybrid orbitals by sp3 hybridization. On three orbitals lone pair of electron is present and fourth orbital forms σ – bond by overlapping with d – orbital of oxygen atom. It’s structure is linear.
Structure of HClO3 :
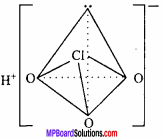
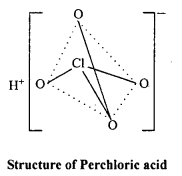
Structure of HClO4 :
sp3 hybridization is found in it’s central atom Cl, hence structure of ClO4 ion is tetrahedral.
MP Board Class 12th Chemistry Important Questions