Students get through the MP Board Class 11th Chemistry Important Questions Chapter 4 Chemical Bonding and Molecular Structure which are most likely to be asked in the exam.
MP Board Class 11th Chapter 4 Chemical Bonding and Molecular Structure
Chemical Bonding and Molecular Structure Class 11 Important Questions Very Short Answer Type
Question 1.
What is a chemical bond?
Answer:
The attraction force between two atoms which binds them to form a molecule is called chemical bond.
Question 2.
What is electronic theory of valency? Write its main postulates. ‘
Answer:
Electronic theory of valency and its main postulates are as follows :
- Valency of an element depends on the number of electrons present in the valence shell of its atom.
- Atoms of all elements possess the ability to achieve stable electronic configuration of inert gas.
- Electrons present in the valence shell of any element are called valence electrons :
When electron is lost from the valence shell, then the remaining vacant orbital is known as Kernel. - If the atom is unstable, it tries to achieve stability. For this it exchange or shares electrons.
On this basis there are three types of bonds :
- Ionic bond,
- Covalent bond,
- Co-ordinate bond.
Question 3.
Write the favourable factors for the formation of ionic bond.
Answer:
The favourable factors for the formation of ionic bond are :
- Ionisation enthalpy of element forming cation should below.
- High negative electron gain enthalpy (electron affinity) of element forming anion.
- High lattice enthalpy of ionic compound formed.
Question 4.
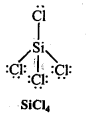
Write Lewis structures of the following molecules and ions :
H2S, SiCl4, BeF2, CO3-2 HCOOH
Answer:
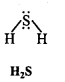

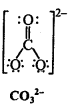
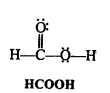
Question 5.
Write Lewis dot symbol of atoms of the following elements :
Mg, Br, Na, O.
Answer:
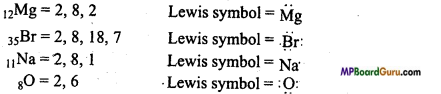
Question 6.
Use Lewis symbols to show electron transfer between the following atoms to form cations and anions
(a) K and S
(b) Ca and O
(c) Al and N.
Answer:
(a) ![]()
(b) 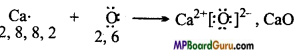
(c) ![]()
Question 7.
What is Lewis symbol? Write Lewis symbol of each of the following elements: Na, Ca, B, Br,Xe, C, N, O
Answer:
Gilburt Newton Lewis utilized the Lewis symbol to represent the valence electrons present in an atom. In this method, electrons present in the valence shell of an atom are represented by dots around the symbol of the element equal to the number of electrons present in the valence shell.
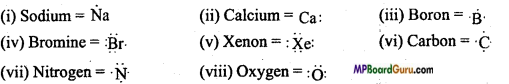
Question 8.
What do you mean by lone pair of electron?
Answer:
Electron pair of an atom which do not take part in bonding is known as lone pair of electron. For example, N in NH3 has one lone pair of electron.

Question 9.
Which molecule show trigonal bipyramidal geometry?
Answer:
Dipole moment is defined as, the product of the magnitude of charge on any one of the atoms and distance between them. It is represented by Greek letter υ(mu).
Mathematically, dipole moment is expressed as
υ = e × d
Where, e is charge on any one of the atoms and d is distance between the atoms.
As e is of the order of 10-10 esu while d is of the order of 10-8 cm υ is of the order 10-18 esu cm and this unit of υ is known as Debye (D).
Thus, 1D = 1×10-18 esu cm.
![]()
Question 10.
Is He2 molecule possible? Justify.
Answer:
He2 molecule is not possible.
2He → 1s2
There are 2 electrons in Is orbital of He atom. It is a complete and stable orbital and cannot accept extra electron. Its bond order is zero, therefore he cannot form molecule.
Question 11.
What is Bond energy?
Answer:
Bond energy of a diatomic molecule, is the energy released when t gm molecule of two neutral atoms are bonded in gaseous state.
A + B → A – B + Energy
In other words, the energy required to break the covalent bond formed between 1 gm molecule of two atoms in gaseous state is called bond energy.
Its unit is Kilo calori per mole or Kilo joule per mole.
Question 12.
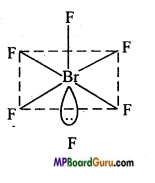
Discuss the geometery of BrF5–.
Answer:
In BrF5–, there are seven electrons in the valence shell of Br (35Br – 2, 8, 18, 7) atom, out of which five electrons form bonds with five fluorine (F) atoms and remaining two electrons, lie as lone pairs. Thus, total 6 pairs (5 bond pair and 1 lone pair) are present. For the minimum repulsion between lone pairs and bond pair its geometry is square-pyramidal
Question 13.
What is the significance of plus and minus sign shown in representing the orbitals?
Answer:
Atomic orbitals are represented in the form of wave functions. In the orbital (+) sign represents positive wave function and (-) sign represents negative wave function. By the combination of two wave functions of similar sign bonding molecular orbital is formed whereas by the combination of two wave functions of opposite signs anti-bonding molecular orbital is formed.
Question 14.
What is the total number of sigma (σ) and pi (π) bonds in the following molecules :
(a) C2H2,
(b) C2H4
Answer:
(a) 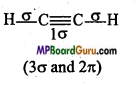
(b) 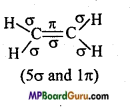
Question 15.
What is hydrogen bond? Write its types and effects.
Answer:
Hydrogen bond is defined as the electrostatic force of attraction which exists between the covalently bonded hydrogen atom of one molecule and the electronegative atom of the other molecule. This bond is represented by dotted line (……………).
It is of two types :
- Inter-molecular hydrogen bond.
- Intra-molecular hydrogen bond.
Question 16.
At normal temperature water is a liquid, whereas hydrogen sulphide is a gas. Why?
Answer:
H2O and H2S are the hydride of oxygen and sulphur lying in the same group but H2O is liquid and H2S is gas, because oxygen is highly electrbnegative so H2O is polar in nature due to this biter-molecular hydrogen Bonding occurs in it. Thus, because of association H2O exists in liquid form and its boiling point is high.
In H2S, due to larger size, electronegativity of S is less, therefore formation of hydrogen bond between H2S molecule is not possible. Their molecules possess only weak van der Waals’ force between them. Thus, H2S is a gas at normal temperature.
![]()
Question 17.
Viscosity of glycerol is higher than ethanol. Why?
Answer:
Ethanol molecule contain one hydroxyl group whereas each glycerol contain three OH group which form three hydrogen bond. That is glycerol has more tendency to form hydrogen bond than ethanol. Therefore, viscosity of glycerol is higher.
Question 18.
Justify the cause of high boiling point and high viscosity of Sulphuric acid.
Answer:
H2SO4 molecule, due to high electronegativity, possess the tendency to form H- bond. Due to H- bond, H2SO4 molecules get associated. Due to this intermolecular hydrogen bond, it does not vaporise easily, therefore, their boiling point and viscosity is high.
Question 19.
What is the cause of solubility of ethyl alcohol in water?
Answer:
Ethyl alcohol easily forms intermolecular hydrogen bond with water molecules. Due to the nature of formation of hydrogen bond, ethyl alcohol is easily soluble in water.

Question 20.
What are the main conditions for the formation of hydrogen bond by a molecule?
Answer:
Following are the conditions for the formation of hydrogen bond by a molecule:
(i) The atom joined by covalent bond to hydrogen atom in a molecule should have very high electronegativity.
(ii) Atomic radius of the atom joined to hydrogen bond should below.
Question 21.
What is meant by Lattice energy and Hydration energy?
Answer:
Lattice energy: Lattice energy of an ionic solid, is the energy released during the formation of one mole solid crystal by the component gaseous ion situated at infinity from each other or in other words, energy released during the formation of a crystal is known as Lattice energy.
Hydration energy: The energy released on dissolving an ion in water or the energy released by the combination of ion with water is called hydration energy.
![]()
Question 22.
What conditions are necessary for the overlapping of atomic orbitals?
Answer:
The following conditions are necessary for the overlapping of atomic orbitals :
- Energy of both the atomic orbitals should be nearly same.
- Nuclear axis of both the atoms should by symmetrical.
- For combination, there should be a definite limit of overlapping i.e, limit of overlapping should be maximum.
Question 23.
What are sigma and pi bond? When are they formed?
Answer:
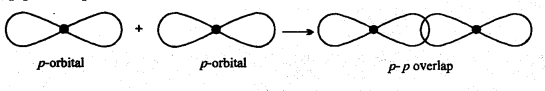
Sigma bond: The bond formed by overlapping of two orbitals along their axis is called a sigma bond. The line joining the two nuclei of the combining atoms is called the intemuclear axis or bond axis.
Example: This type of overlapping takes place between s-s orbital, s-p orbital and px – px orbitals.
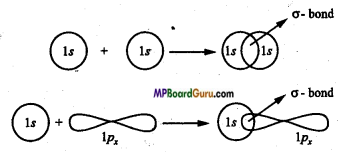
Question 24.
Why is σ-bond stronger than π-bond?
Answer:
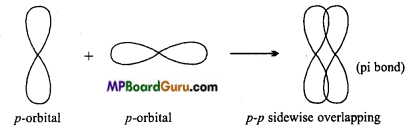
Strength of bond depends upon the extent of overlapping. In σ-bond formation, the extent of overlapping is more because overlapping takes place along the inter-nuclear axis while in case of π -bond formation extent of overlapping is less due to sidewise overlapping, hence σ-bond is stronger than π-bond.
Question 25.
Write the differences between atom and ion.
Answer:
Differences between Atom and Ion
| Atom | Ion |
| 1. Atoms are electrically neutral. | Ion is electrically charged. |
| 2. Cannot exist independently. | Ion can exist in independently. |
| 3. Atom participates in chemical reaction. | Ions do not participate in chemical reaction. |
| 4. Number of electrons and protons are same. | Number of electrons is more or less than the number of protons. |
Question 26.
Differentiate Sigma (σ) and Pi (π) bond.
Answer:
Differences between Sigma (σ) and Pi (π) bond
| Sigma (σ) bond | Pi (π) bond |
| 1. This bond is formed by end to end or head on overlapping of orbitals along the internuclear axis. | This bond is formed by the side-wise overlapping of orbitals. |
| 2. This is formed by overlapping of s-s, s-p or p-p orbitals. | This is formed by overlapping of p-p orbitals only. |
| 3. Overlapping is large, hence it is strong bond. | Overlapping is small, hence it is weak bond. |
| 4. Free rotation about a σ – bond is possible. | Free rotation about a π-bond is not possible. |
| 5. Electron cloud is symmetrical about internuclear axis. | Electron cloud of π-bond is unsymmetrical. |
Question 27.
HCl is a covalent compound, still its aqueous solution gets ionized.
Answer:
In HCl due to high electronegativity of chlorine than hydrogen the shared electron pair gets displaced towards Cl and a partial negative charge is developed, on Cl and partial positive charge is developed on H. As a result, when HCl is dissolved in water, then due to partial polarizability HCl gets ionized.
Question 28.
Among electro-valent anti covalent compounds, whose boiling point is higher and why?
Answer:
Crystals of electro-valent compounds are formed by the combination of ions and strong electrostatic force is present between these ions and high energy is required to separate these ions and vaporize them. Therefore, boiling point of electro-valent compounds is higher than covalent compounds because covalent compounds are formed by the combination of atoms and only weak van der Waal’s force is present between them.
![]()
Question 29.
BaSO4 is insoluble in water. Why?
Answer:
Solubility of an ionic compound in water depend on Lattice energy and hydration energy. If lattice energy of a compound is more than its hydration energy, then the ionic compound is insoluble in water. Magnitude of Lattice energy of BaSO4 is higher than its hydration energy, therefore, BaSO4 is insoluble in water.
Question 30.
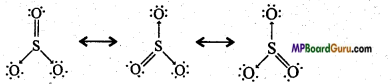
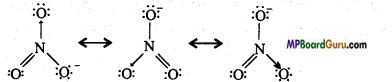
Write the resonance structures of SO3, NO2, and NO3–.
Answer:
(SO3)
(NO2)

(NO3–)
Question 31.
Sodium is a strong electropositive element. Why?
Answer:
Atomic number of Sodium is 11 and its electronic configuration is 2,8,1. Due to large size, its ionization energy is very less, so it easily loses electron and forms a positive ions. Therefore, is a strongly electropositive element.
Question 32.
Electrovalent bond is formed between which type of compounds?
Answer:
Ionic bond is formed between strongly electropositive and strongly electrovalent elements because strongly electropositive element easily lose electron and forms cation and electronegative element easily gains electron and forms anion.
Strong electrostatic force of attraction is present between cation and anion. This strong electrostatic force is called electro valent or ionic bond.
Example : Na2,8,1+ Cl2,87 → Na+28+Cl–2,8,8.
Question 33.
HF molecule is more polar than HI molecule. Why?
Answer:
Electronegativity of Fluorine is more than Iodine, therefore, displacement of shared electrons in HF is more than in HI, as a result division of charge is more in HF, then HI therefore, HF is more polar than HI.
![]()
Question 34.
C-Cl bond is polar, but CCl4 is non-polar. Given reason.
Answer:
In C-Cl bond, electronegativity of Chlorine is more than in C, due to which shared electrons gets displaced more towards Chlorine, by which partial positive charge is developed on C and partial negative charge is developed on Cl and the bond represents polar nature.
Whereas, CCl4 has symmetrical structure due to which dipole moment of C-Cl bonds cancel each other therefore, CCl4 molecule is non-polar.
Question 35.
What do you mean by resonance?
Answer:
When properties of a molecule are not explained by one structure and two or more than two structures are assigned to express its characteristics, it is said that molecule is resonance hybrid of these structures and this property is known as resonance.
Different resonating structures are exhibited by using sign (↔) in between these structures.
Question 36.
What are the main conditions of Resonance?
Answer:
Conditions for resonance are as follows :
- Enthalpy of formation of all resonating forms is nearly same.
- In every formula, arrangement of atoms should be same.
- Number of unpaired electrons in all the resonating forms should be equal.
Question 37.
What do you understand by Resonance energy?
Answer:
There is a difference in actual energy of a molecule and the energy calculated by its formula. This difference between both these energies is called resonance energy. In other words, difference between resonance hybrid and most stable resonance structure is called resonance energy. Higher the value of resonance energy, more is the stability of the molecule.
![]()
Question 38.
Calculate the bond order of N2.
Answer:
Electronic configuration of N2( 14 electron):
![]()
Bond order = \(\frac{1}{2}\) [Nb -Na] = \(\frac{1}{2}\)[10-4] = 3
Question 39.
On the basis of molecular orbital theory explain that Be2 molecule has no existence.
Answer:
Electronic configuration of 4Be = 1s2,2s2
Electronic configuration of 4Be molecule (4 + 4 = 8e–)
= ![]()
Bond order = \(\frac{1}{2}\)[Nb-Na] = \(\frac{1}{2}\)[4-4] = 0
Thus, Be2 molecule has no existence.
Question 40.
What is hybridization?
Answer:
Hybridization: The hybridization may precisely be defined as the concept of mixing or merging of orbitals of an atom having equal energy to produce entirely new orbitals (equal number to the mixing orbitals) which have same energy contents, identical shapes and symmetrically disposed in space.
Question 41.
Write the differences between s-orbital and p-orbital.
Answer:
Differences between s-orbital and p-orbital
| s-orbital | p-orbital |
| 1. These orbitals are spherically symmetrical. | 1. These orbitals are dumb-bell in shape and are symmetrical on their axis. |
| 2. These are non-directional. | 2. These are directional. |
| 3. For these l=0 and m = 0. | 3. For these l = 1 and m = -1, 0, +1. |
Question 42.
Boiling point of water is higher than HF whereas electronegativity of Fluorine is more than oxygen. Explain.
Answer:
In a molecule of HF one Fluorine is linked to one H, due to which each HF molecule can form two hydrogen bonds whereas in H2O molecule, oxygen atom is linked to two H– atom due to which each water molecule can form four hydrogen bond. This way, tendency to form H-bond in water is more than in HF. Thus, boiling point of water is higher than HF.
![]()
Question 43.
Write the properties of Co-ordinate compounds.
Answer:
Properties of Co-ordinate compounds :
- Co-ordinate compounds generally soluble in water and soluble organic solvents.
- Their melting and boiling points are higher than covalent compounds and lower than electrovalent compounds.
- They do not ionize on dissolving in water or in other solvents.
- These bonds like covalent bonds are directional thus these compounds also show stereoisomerism.
Question 44.
Write the type of geometrical shapes and value of bond angle of the following molecules:
(i) C2H2
(ii) BCl3
(iii) NH3
(iv) PCl5.
Answer:
Types of geometrical shapes and value of Bond angle :

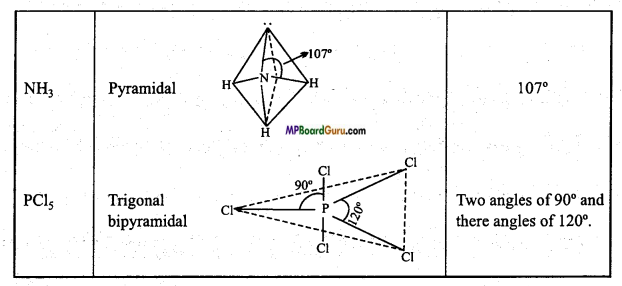
Question 45.
Draw the resonating structure of the following:
(i) ![]()
(ii) CO2.
Answer:
(i) Resonating structure of ![]() :
:
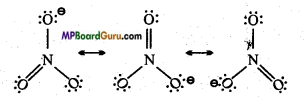
(ii) Resonating structures of CO2:
![]()
Chemical Bonding and Molecular Structure Class 11 Important Questions Short Answer Type
Question 1.
On what factors does formation of ionic compound depend?
Answer:
Formation of ionic bond depends on the following factors :
- Electronegativity: There should be very high difference in electronegativity be¬tween the two combining atoms. Higher the difference, higher will be the ionic property of the bond.
- Ionisation energy : Ionisation energy of the atom donating electron should be very low, due to which the atom can easily lose electron and form cation.
- Electron affinity: Electron affinity of the atom gaining electron should be high, due to which the atom can easily gain electron.
- Lattice energy: Higher the lattice energy of the compound higher will be its ionic nature.
Question 2.
Write the characteristics of hybridization.
Answer:
Characteristics of hybridization :
- The orbitals taking part in hybridization belongs to same atom.
- The orbitals which participate in hybridization must have a small difference of energies.
- Both half filled and completely filled orbitals can take part in hybridization.
- Only orbitals participate in hybridization not electrons.
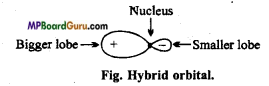
- Number of hybrid orbitals formed is equal to the number of atomic orbitals hybridized.
- Hybrid orbitals have equivalent energies and identical shape. They may have different orientation.
- Hybridization is a concept, which is used to justify the experimental facts.
Question 3.
How a covalent bond is formed? Explain by giving examples.
Answer:
Covalent bond: The bond formed by mutual sharing of electrons between the atoms is covalent bond. The compound obtained as a result of such bonding is called cova¬lent compound. When one-one electron is shared between two atoms, single bond is formed which is shown by line (-).
When two-two electrons are shared between two atoms results in the formation of double bond and is expressed with (=) line. Similarly, sharing of three electrons between two atoms forms triple bond expressed with (≡) line.
Example: Formation of Cl2: Electronic configuration of chlorine is 2, 8, 7. Its valence shell contains 7 electrons. It needs one electron to complete its octet. One chlorine atom shares its electron with other chlorine atoms to complete their octet.
In this way, in one chlorine molecule (Cl2) one single bond is formed between chlo¬rine atoms.
![]()
Question 4.
What is the difference between polar and non-polar covalent bonds?
Answer:
Polar covalent bond: The molecules formed of unlike atoms which differ in their electronegativity value contain polar bonds e.g., HCl, NH3, HF, H2O etc. as their bonds have ionic nature also.
Example: Hδ+ — Clδ- is a polar molecule.
Non-polar covalent bond: The covalent bond that have no ionic character are called non-polar bonds. The molecules which are formed of like atoms contain non-polar bonds. e.g., H2, N2, O2 etc. are a non-polar molecule.
H:H ![]()
Question 5.
Write characteristics of covalent compounds.
Answer:
Following characteristics of covalent compounds :
- Generally these compounds are in gaseous or liquid state. Solids are of high molecular weight.
- Crystal lattice consist of neutral molecules or atoms.
- Boiling points and melting points are low.
- These are bad conductor of electricity.
- Reactions of these compounds are slow being molecular reactions.
- These compounds are soluble in non-polar solvents like benzene, pyridine, ether etc.
- Covalent bonds are rigid and directional. So many types of isomerism is found.
Question 6.
Differentiate Electrovalent, Ionic compound and Covalent compound.
Answer:
Differences between Ionic and Covalent compounds
| Property | Ionic compound | Covalent compound |
| 1. Formation | These are formed by the transfer of electrons. | These are formed by the sharing of electrons. |
| 2. Reactivity | More reactive. | Less reactive. |
| 3. Solubility In water | Completely soluble. | Partially soluble or in soluble. |
| 4. Ionization | Ionizes in aqueous solution. | Do not ionize in water. |
| 5. Electrical conductivity | Conductor of electricity in molten state or in aqueous solution. | Non-conductor of electricity. |
| 6. Melting point & boiling point. | High melting point and boiling point. | Low melting point and boiling point. |
| 7. Reaction rate | Reaction rate is fast in solution state. | Reaction rate is slow. |
Question 7.
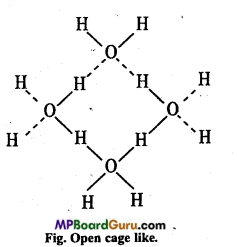
Ice is lighter than water. Why? Explain. Or, Density of ice Is less than water. Why?
Answer:
In ice each oxygen atom is tetrahedrally surrounded by four hydrogen atoms in which two hydrogen atoms are linked to oxygen atom by covalent bond and other two hydrogen atoms are linked by hydrogen bond.
The molecules of H2O are not packed closely. This gives rise to open cage-like structure for ice having a
larger volume for thé given mass of water.
Thus density of ice is less than water. Ice is actually hydrogen-bonded crystal. Three-dimensional structure of protein and H ucleic acids like biologically important substances is due to hydrogen bond. Energy of hydrogen bond is between 35 kJ mol-1 and 8 kJ mol-1. Thus, hydrogen bond is stronger than Van der Wall’s force and weaker than covalent bond.
Question 8.
Explain co-ordinate bond with example.
Answer:
Coordinate bond is also formed by the sharing of electrons between two atoms but the shared electron pair is donated by one atom only whose outer orbit is already stable the other atom gains that electron pair and both the atoms achieve stable configuration.
Atom which donates electron is called donor atom and the atom which accept electron is called acceptor. Coordinate bond is represented by an arrow (→) which starts from the atom which donates electron pair and points to the atom which accepts them. Example: In the combination of ammonia and Boron trifluoride N atom donates its electron pair to B
atom forming a co-ordinate bond.
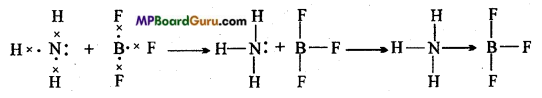
Question 9.
Is there any change in the hybridization of B and N atoms as a result of the following reaction:
BF3 + NH3 → F3B.NH3
Answer:
In BF3 there are 3 bond pairs and 0 lone pair due to which Boron is sp2 hybridized and in NH3 due to the presence of 3 bond pairs and 1 lone pair Nitrogen is sp3 hybridized. After the reaction hybridization of Boron changes to sp3 but hybridization of nitrogen remains same because N shares its lone pair of electron with electron-deficient B.
![]()
Question 10.
Describe the change in hybridization (if any) of the A1 atom in the following reaction:
AICI3 + Cl– → AICI3–
Solution:
Electronic configuration of Al is as follows :
In ground state configuration 13Al – 1s2, 2s22p6,3s23px1 .
In excited state – 1s2, 2s22p6,3s23px13py1
In the formation of AlCl3, Al is sp2-hybridized and its geometeiy is trigonal planar. On the other side in the formation of AlCL4– due to participation of vacant 3pz orbital, A1 becomes sp3-hydrized and its geometry is tetrahedral.
Question 11.
What is metallic bond? Explain with example.
Answer:
To explain the metallic bond, there are two theories of the bond present in metals:
1. Independent (Free) electron theory: This theory was proposed in 1990 by Drud and modified later in 1916 by Lorenz. According to this theory, metallic solid is that group of cations which are orderly immersed in a sea of mobile electrons and the force which bonds these cations in the electron sea is called metallic bond.
Example: Each atom of Mg metal donates 2 electron to form lattice of Mg2+ ion and electron sea is formed by valence electrons.
2. Valence bond theory: According to this theory “Bond in metals is of covalent nature and resonance is found in it.”
Example: Each Li atom is surrounded by eight other Li atoms and it forms covalent bond with each Li turn by turn. This way, various resonance structures are obtained.
Question 12.
What do you understand by intermolecular and intramolecular hydrogen bonding?
Answer:
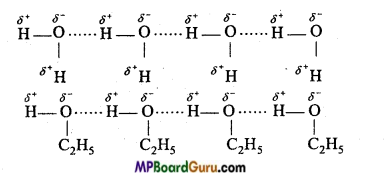
(i) Intermolecular hydrogen bonding: When these atoms (hydrogen and electronegative atom) are of different molecules, it is called intermolecular hydrogen bonding as in H2O, HF, C2H5OH etc.
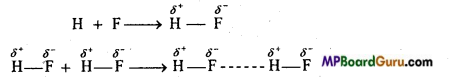
Hydrogen bond is represented by dotted lines. Many molecules of HF associates and form (HF)n. On the same way molecules of water and alcohols are linked with hydrogen bonds.
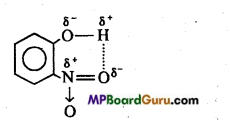
(ii) Intramolecular hydrogen bonding: If these atoms (hydrogen and electronegative atoms) are present in same molecule, this type of hydrogen bonding is called intramolecular hydrogen bonding.
Question 13.
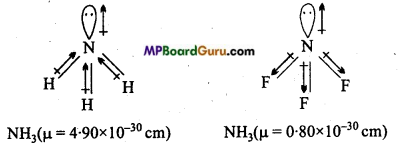
Which out of NH3 and NF3 has higher dipole moment and why?
Answer:
Shape of both NH3 and NF3 is pyramidal, but dipole moment of NH3 is higher than NF3. In NH3, dipole of lone pair electron is in the direction of three N-H bond, where as dipole of lone pair in NH3 is in opposite direction of three N-F bond due to which magnitude of dipole moment of NF3 decreases.
Question 14.
Explain why BeH2 molecule has zero dipole moment although the Be-H bonds are polar?
Answer:
BeH2 molecule is linear. Two similar bond dipoles in opposite directions cancel the effect of each other. That is why, dipole moment of BeH2 molecule is zero.
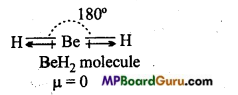
Question 15.
What are the main postulates of atomic orbital overlapping of covalent bond?
Answer:
According to this theory:
- A covalent bond is formed by the overlapping of atomic orbital of one atom with the orbital of the other atom.
- At the formation of bond, electrons are related to the nuclei of both the atoms.
- Spin of the bonded electron should be in opposite direction.
- Strength of bond depends on the extent of overlapping.
- On the basis of overlapping bonds are of two types : a bond and n bond.
- Valency of an element depends on the number of unpaired electrons present in its n valence shell.
- Overlapping takes place only in those orbitals which takes part in bond formation.
- Multiple bonds are formed by the overlapping of orbitals containing more than one unpaired electrons.
Question 16.
Explain the cause and arrangement of the following compounds in the increasing order of their ionic behaviour : N-H, F-H, C-H and O-H
Answer:
Higher the electronegativity difference between two bonded atoms in a molecule higher is the ionic behaviour.
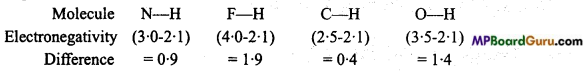
Thus, correct order of ionic behaviour is as follows : C-H < N-H < O-H < F-H.
![]()
Question 17.
Justify: Why is PCl5 trigonal bipyramidal and IF5 is square pyramidal?
Answer:
PCl5: In it, Phosphorus (Z = 15) is the central atom whose electronic configuration in ground state and excited state is as follows :
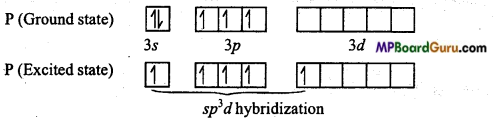
In PCl5, Phosphorus is sp3d hybridized, thus its geometry is trigonal bipyramidal.
IF5: In it, central atom is iodine (Z = 53), whose ground state and excited state elec¬tronic configuration is as follows :
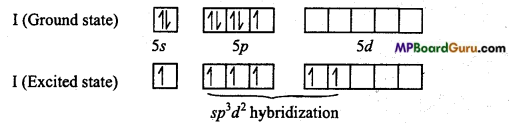
In IF5, Iodine is sp3d2 hybridized, thus its geometery is square pyramidal, distortion is due to lone pair electron.
Question 18.
What are sigma (σ) and pi (π) bonds? When are they formed? Explain.
Answer:
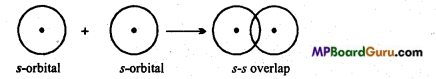
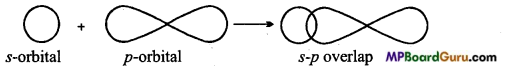
Sigma (σ) bond: Sigma bond is formed by axial or end to end overlapping of orbitals. In sigma bonds, electron density is maximum at intemuclear axis due to maximum overlapping so sigma bond is stronger than π-bond. Formation of sigma bonds occur on s- s, s-p and p-p overlapping e.g.,
(i) s-s overlap :
(ii) s-p overlap:
(iii) p-p overlap :
Pi (π) Bond: Bond formed by the lateral or side wise overlapping of two p-orbitals is called π-bond. It is a weak bond.
Question 19.
Tell the hybridization and geometry of the following compounds on the basis of VSEPR theory: SnCl2, SiCl4, SF6, H2S, HF, NCl3, PCl5.
Answer:
Hybridization and geometry of the given compounds :
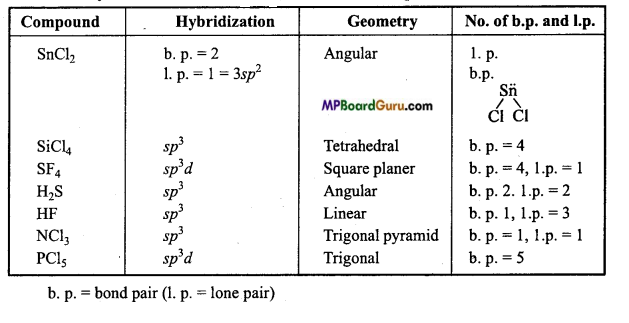
Question 20.
Which hybrid orbitals are used by carbon atoms in the following molecules: (a) CH3-CH3 (b) CH3-CH = CH2
(c) CH3CH2-OH
(d) CH3-CHO
(e) CH3COOH.
Answer:
(a)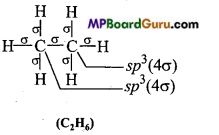
(b) 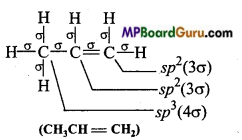
(c) 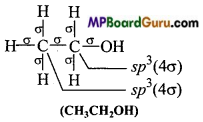
(d)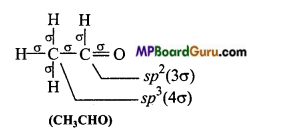
(e) 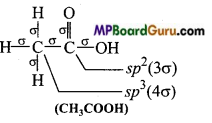
Question 21.
What do you understand by bond pairs and lone pairs of electrons ? Illustrate by giving one example of each type.
Answer:
Covalent bond is formed by the mutual sharing of electrons. Electron pairs shared between bonded atoms are called bond pair. Electrons which do not participate in bond formation are called lone pair.
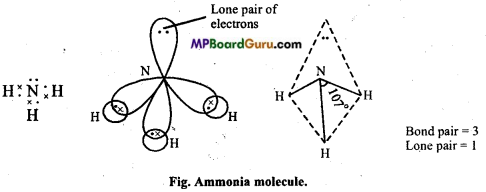
Example: In ammonia (NH3), three bond pairs and one lone pair electron are present.
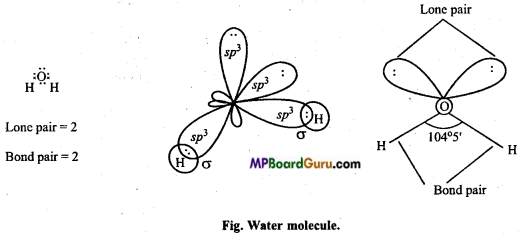
In water (H2O), two bond pairs and two lone pairs electrons are present.
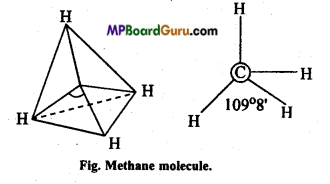
In Methane only four bond pair electrons are present.
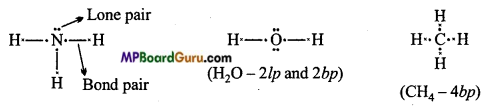
Question 22.
What is s-s overlapping? Explain with example.
Answer:
When half filled s-orbital of an atom overlaps with half-filled s-orbital of the other atom σ bond is formed, then it is known as s-s overlapping. This bond is symmetrical along the nuclear axis.
Example: Formation of H2 molecule: Atomic number of H-atom is 1. Its normal electronic configuration is 1.s1. During the formation of H2 molecule half-filled orbital of H- atom, overlaps with half-filled orbital of other H-atom to form a bond.
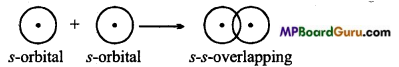
Question 23.
What is s-p overlapping? Describe with example.
Answer:
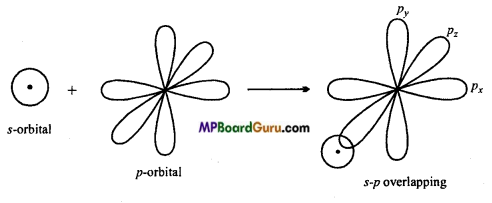
s-p overlapping: When half filled s-orbital of any atom overlap with p-orbital (half-filled) of other atoms, this type of overlapping is called s-p overlapping. Molecular orbital thus formed is called s-p molecular orbital. Bond formed by this overlapping is a (sigma) bond. 5-orbital is non-directional or spherical so it can be overlap with Px, py or pz
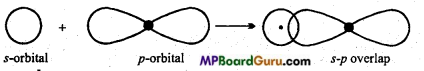
Example: Formation of HCI: Electronic configuration of hydrogen and chlorine atoms are:
1H = 1s1
17Cl= 1s2,2s22p6,3s2 3p2x3py2 3pz1
Thus, 1s-orbital of hydrogen atom overlap with 3p2 orbital of chlorine atom and form a s-p molecular orbital and HCl molecule is formed.
Question 24.
Write Lewis sü .cture of the following compound and express formal charge on each atom.
(a) HNO3,
(b) NO2,
(c) H2SO4.
Answer:
(a) HNO3:
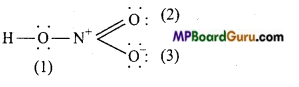
Formal charge on H =1-0 – \(\frac{1}{2}\) – × 2 =0
Formal charge on N =5-0 – \(\frac{1}{2}\) × 8 = 1
Formal charge on O(1) =6-4- \(\frac{1}{2}\) × 4 = 0
Formal charge onO(2) =6-4- \(\frac{1}{2}\)×4=0
Formal charge on 0 (3) = 6-6- \(\frac{1}{2}\) ×2 = -1.
(b)NO2:
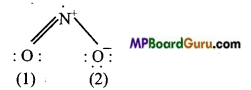
Formal charge on 0 (1) =6-4- \(\frac{1}{2}\) × 4 = O
FormalchargeonN =5-1- \(\frac{1}{2}\)×6=+1
Formal charge on 0(2) =6-6-\(\frac{1}{2}\) × 2= -1
(c) H2SO4:
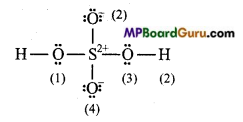
Formal charge on H (1) and H (2) =1-0 – \(\frac{1}{2}\) × 2 =0
Formal charge on 0(1) and 0 (3) =6-4- \(\frac{1}{2}\) × 4 =0
Formal chargeonO(2) and 0(4) =6-6- \(\frac{1}{2}\)× 2=-1
Formal charge on S =6 – 0- \(\frac{1}{2}\) × 8 = +2.
![]()
Question 25.
Justify the cause of difference in the properties of the two aiiotropes diamond and graphite.
Answer:
Diamond and graphite, both are the allotropes of carbon but due the difference in arrangement of C, their properties are different. In diamond, C-atom is in sp3 hybrid state and each C-atom is linked with other four carbons and forms tetrahedral structure. This way, it forms a three-dimensional structure, therefore diamond is hard and its melting point is high.
In graphite, each C-atom is in sp2 hybrid state i.e. each C-atom is surrounded by three Other carbon and fourth valency of each C-atom is unsaturated. There are various layers in graphite which are joined by weak van der Waals’ attractive force. Therefore, graphite is soft and due to the presence of free electrons, it is a good conductor of electricity.
Question 26.
Explain the hybridized structure of acetylene molecule with figure.
Answer:
Explanation of acetylene by sp hybridization: In this molecule, carbon is the central atom. Thus, both the carbon atom assumes sp hybridization as shown below :
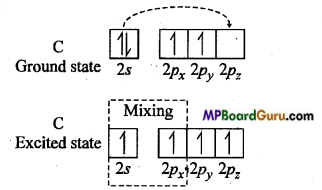
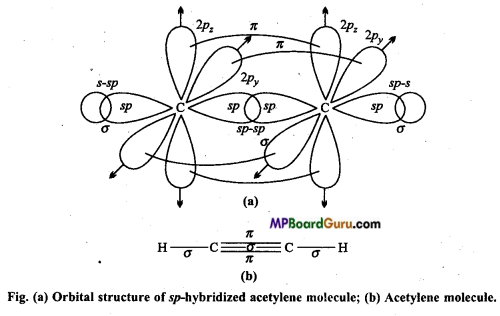
One 2s and one 2p -orbital of each carbon atom hybridizes while other two 2p-orbitals are left unhybridized. The two sp hybrid orbitals formed by each carbon atom are oriented linearly while unhybridized 2pz orbitals are oriented perpendicularly to hybrid orbitals. During the formation of acetylene molecule one sp hybrid orbital of each carbon atom overlaps forming carbon-carbon sp-sp sigma bonds.
Remaining two sp hybrid orbitals of both the C-atoms overlaps with half-filled 1s orbital of H-atom to form two carbon-hydrogen sp-s sigma bonds. Each unhybridized orbital overlaps sidewise forming two Pi (π) bonds. Thus, sp hybrid linear acetylene molecule containing two π-bonds and three sigma bonds is formed. In this molecule, C≡C bond length is 120 pm while C-H bond length is 100 pm. H-C-C bond angle is 180°.
Question 27.
Considering X-axis as the internuclear axis which out of the following will not form a sigma bond and why :
(a) 1s and 1s
(b) Is and 2px
(c) 2py and 2py
(d) 1s and 2s.
Answer:
Only (c) will not form carbon, because on taking X-axis on internuclear axis, 2py and 2py undergo lateral overlapping in between the axis, as a result of which π bond is formed.
Question 28.
Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H-atoms at the corners of the square and C at its centre. Explain why CH4 is not square planar?
Answer:
Electronic configuration of C is as follows:
In ground state 6C = 1s2,2s2,2px1, 2py1,2pz0
In excited state 6C = 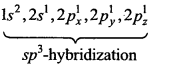
In CH4 molecule, C is sp3-hybridized due to which its geometry is tetrahedral. For square planar geometry dsp2 hybridization is required. But due to absence of rf-orbitals in carbon, it is not possible. Along with this, according to VSEPR theory, four bond pairs of carbon atom are situated in tetrahedral geometry.
In tetrahedral structure, bond angle is 109°28′ and in square planar geometry is 90°. Thus, repulsion of bonded electrons in tetrahedral geometry is less as compared to square planar geometry.
Question 29.
Justify on the basis of hybridization, that structure of BeCl2 molecule is linear.
Answer:
In BeCl2, Be is in sp-hybrid state.
sp-hybridization: When one s-orbital and one orbital of p-subshell redistribute their energy to form two hybrid orbitals, it is known-as sp-hybridization. Shape of BeCl2 molecule : In BeCl2, B is the central atom, its normal oxidation state is 1s2,2s2,2p0.
In excited state one electron of 2s orbital gets excited and goes to vacant 2p subshell. One orbital of 2s and one orbital of 2p redistribute their energy to form two hybrid orbitals which arrange themselves making an angle of 180°. Both these hybrid orbitals overlap with two chlorine atoms to form two a bonds. Therefore structure of BeCl2 is linear.
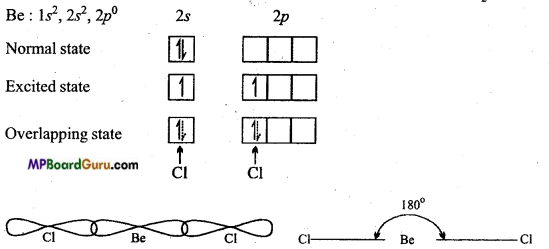
Question 30.
What is sp3 hybridization? Justify with example.
Answer:
sp3-hybridization: When s-orbital and three orbitals of. p-subshell mutually redistribute their energy and form four hybrid orbitals, then it is known as sp3-hybridization.
Due to repulsion, all these four orbitals form an angle of 109°28′ with one another i. e., these four orbitals directed towards the four comers of a tetrahedron.
Example: CH4: Atomic number of carbon is 6 and its normal electronic configuration is 1s2, 2s2, 2p2.
In excited state, one electron of 2s gets excited and moves to 2pz orbital. Thus, in excited state C has four unpaired electrons. 2s orbital of carbon and all the three orbitals of 2p subshell redistribute their energy and form four hybrid orbitals which overlaps with four hydrogen atoms to form a bond. Due to sp3-hybridization, structure of methane molecule is tetrahedral and bond angle is 109°28′. ‘
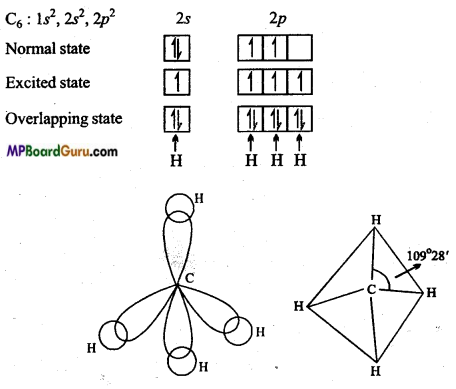
Question 31.
Explain the structure of HNO3 and H2SO4.
Answer:
Structure of HNO3 :

Structure of H2SO4
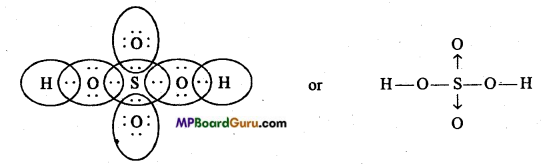
Question 32.
Write the conditions for the formation of molecular orbitals by Linear Combination of Atomic Orbitals.
Answer:
The following conditions are required for the formation of molecular orbitals by Linear Combination of Atomic Orbitals: ‘
(i) The combining atomic orbitals should possess same or nearly similar energy. For example:1s-orbital can combine with 1s-orbital, not with 2s because energy of 2s-orbital is more than 1s-orbital. This way, combination is possible when the combining atoms are different (Intemuclear diatomic molecule).
(ii) The combining atomic orbitals must have the same symmetry about the molecular
axis.
(iii) Combining atomic orbitals should undergo maximum overlapping. Higher the overlapping between the orbitals, higher the electron density between their nuclei.
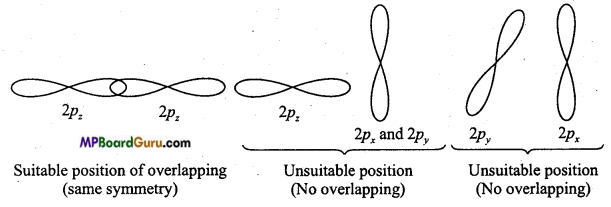
Question 33.
Explain the formation of N2 molecule on the basis of orbital theory or overlapping.
Answer:
Atomic number of N-atom is 7, it normal electronic configuration is 1s2,2s2,2p3. It has three electrons in the p-subshell of its valence shell and to complete its octet, it requires three more electrons. px orbital of N-atom, overlaps with pxorbital of another N-atom and form a bond and p2 orbital of both nitrogen form two n bonds by lateral overlapping. This way three bonds are formed between both N-atoms in N2 molecule.

Question 34.
Write the main points of Molecular Orbital Theory.
Answer:
Main points of Molecular Orbital Theory :
- Molecular orbitals are formed by the combination of atomic orbitals of atoms.
- It is supposed that molecular orbitals are formed by the linear combination of atomic orbitals.
Molecular orbitals are of two types :
- Bonding molecular orbitals: These are formed by addition of wave functions.
- Antibonding molecular orbitals: These are formed by the difference of wave functions.
- Molecular orbitals are polycentric, thus electrons are effected by two or more than two nuclei.
- Atomic orbitals of inner shells (apart from valence shell) also combine to form molecular orbitals. These are normally called nonbonding molecular orbitals. Molecular orbitals are formed by overlapping.
Filling of electrons follow Aufbau’s principle, Hund’s Rule and Pauli’s principle.
![]()
Question 35.
Write the hybridization, geometry and bond angle present in some specific compounds.
Answer:
Hybridization, geometry and bond angle in compounds :
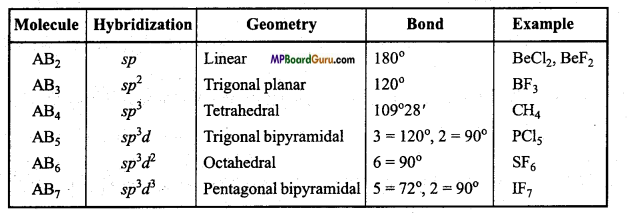
Chemical Bonding and Molecular Structure Class 11 Important Questions Long Answer Type
Question 1.
State important applications of dipole moment.
Answer:
Important applications of dipole moment are as follows :
- By the help of dipole moment, polar and non-polar nature of a molecule can be determined. Higher the magnitude of dipole moment (υ = q × d), higher is its polarity. For non-polar molecules, magnitude of dipole moment is zero. For example: CO2.
- By its help, percent ionic character can be determined as follows :
Percent ionic character = \(\frac{\mu \text { observed }}{\mu \text { ionic }}\) × 100 - Dipole moment of Symmetric molecules is zero, though they contain two or more polar bonds. Thus, used in measuring the symmetry of molecules.
- By its help, cis and trans isomers can be differentiated. Normally, dipole moment of cis isomer is higher than that of trans isomer.
- It is also used in the differentiation of ortho, meta and para isomers. Dipole mo¬ment of para isomer is zero. Dipole moment of ortho isomer is more than meta isomer.
- Information regarding shape of molecule is obtained.
Question 2.
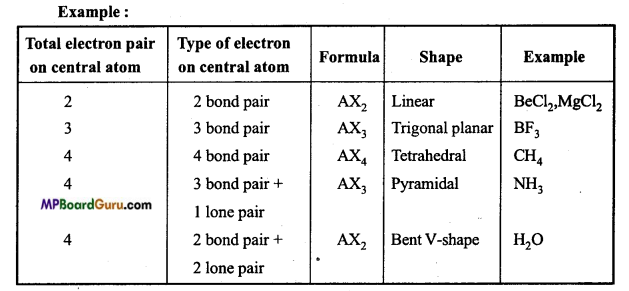
What is valence shell electron pair repulsion theory? State its limitations. Or, Explain valence shell Electron pair repulsion theory with example.
Answer:
Geometry of covalent molecules can be justified on the basis of VSEPR theory. According to this theory:
Geometry of a molecule depends on the number of bonded and lone pair electron or non-bonding electron in valence shell of central atom.
If only bonded electron pairs are present in the valence shell of central atom of a molecule, then the geometry of the molecule is symmetrical and depends on the type of hybridization of valence shell contain 2,3,4,5,6 and 7 bond electron pair the geometry of the molecule is linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral and pen¬tagonal bipyramidal.
If valence shell of central atom of molecule contains both bond and lone pair electrons, then the geometry of the molecule is not symmetrical because due to the presence of electron pair, these is more repulsion between bond and lone pair electrons which destroy the geometry. Order of repulsion in various electron pair is as follows :
Lone pair – Lone pair > Lone pair – Bond pair > Bond pair – Bond pair.
Due to increase in the number of lone electron pairs on central atom, magnitude of bond angle between bonded electron pair decreases.
If valence shell of central atom is fully filled with electrons then repulsion in bonded electron pair is more than the repulsion by bonded electron pair in incomplete va¬lence shell.
Limitations :
- On the basis of this theory, geomtry of complex salts of transition metals cannot be explained.
- By the help of this theory, geometry of such molecules which have highly displaced π-electrons cannot be explained. Shape of highly polar compound like Li2O cannot be discussed.
Question 3.
Compare the relative stability of the following species and indicate their magnetic properties:
O2,O2+,O2– (Superoxide), O2–(Peroxide).
Answer:
Electronic configuration of species is as follows :
(a) O2(16c–) → 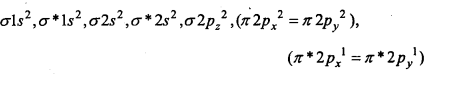
Bond order = \(\frac{1}{2}\) (Nb – Na ) = \(\frac{1}{2}\) (10 – 6) = 2
Thus, 0 = 0
Due to the presence of two unpaired electrons, O2 is paramagnetic.
(b) O2+ ion is formed by the removal of one electron from O2 molecule
O2 → O2+ + e–
O2+ (15 e–) →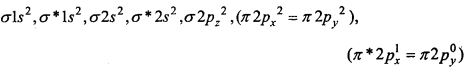
Bond order = \(\frac{1}{2}\) (Nb-Na)= \(\frac{1}{2}\) (10-5) = 2.5
InO2+ one electron is unpaired, thus it is paramagnetic.
(c) O2– (Superoxide) ion : O2– is formed by the addition of one electron in O2 molecule.
O2 + e → O2
O2– (17e–) → 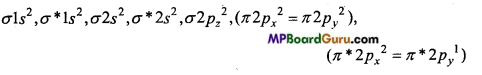
Bond order = \(\frac{1}{2}\)(Na – Nb) = 1(10-7) = 1.5
Due to the presence of one unpaired electron, O2– is paramagnetic.
(d) O22- (Peroxide) ion : O22- ion is formed by the addition of two electrons in O2 molecule.
O2+2e– → O22-
O22- (18e–) → 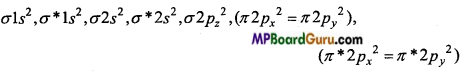
Bond order =\(\frac{1}{2}\) (Nb-Na) = \(\frac{1}{2}\)(10-8) =1
Due to presence of paired electrons O22- is diamagnetic.
Decreasing order of stability : We know that, Bond order « Stability.
Thus, decreasing order of stability is as follows :
O2+ > O2 > O2– > O22-.
![]()
Question 4.
What is hydrogen bond? Write its types. How does it affect the properties of compounds?
Answer:
Hydrogen bond is defined as the electrostatic force of attraction which exists between the covalently bonded hydrogen atom of one molecule and the electronegative atom of the other molecule. This bond is represented by dotted line (….). Hydrogen bond exists between H-atom of one molecule and electronegative (O, N, F) atom of another molecule:
Example : HF: ![]()
It is a weak bond.
Types of hydrogen bond : Hydrogen bond is defined as the electrostatic force of attraction which exists between the covalently bonded hydrogen atom of one molecule and the electronegative atom of the other molecule. This bond is represented by dotted line (……………).
It is of two types :
- Inter-molecular hydrogen bond.
- Intra-molecular hydrogen bond.
Effects of hydrogen bond: Hydrogen bond affects the physical and chemical properties like boiling point, solubility etc. of compounds. Due to intermolecular hydrogen bond, molecules get associated, therefore their melting and boiling points are high whereas due to intramolecular hydrogen bond melting point, boiling point and solubility etc. of compound becomes low.
Example:
1. Physical state: In H2O, due to hydrogen bond, molecules of water are in associated state, therefore at normal temperature H2O is a liquid whereas due to low
electronegativity of S, hydrogen bond in H2S is not possible. Their molecules possess weak van der Waals’ force, therefore it is a gas at normal temperature.
2. Solubility: Alcohol and carboxylic acid easily form intermolecular hydrogen bond, therefore they are soluble in water.
Question 5.
Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds ?
Answer:
Electronic configuration of phosphorus is as follows :
15 P = 1s2,2s2 2p6,3s2,3p3
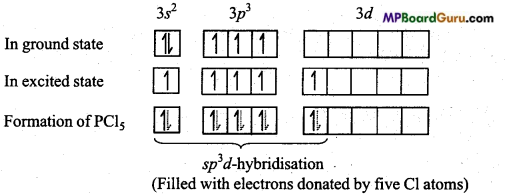
In the formation of PCl5 molecule, one s, three p and one rf-orbital are available for hybridisation. These orbitals combine to form five sp3d hybrid orbitals. These five hybrid orbitals are oriented towards the five comers of a trigonal bipyramid.
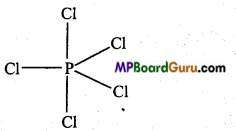
Again, in PCl5 three P – Cl bonds are situated on one plane and form an angle of 120°. These are known as equatorial bonds, remaining two P – Cl bonds lie above and below the equatorial bonds making an angle of 90°. These bonds are called axial bonds. Since axial bond feel more repulsion than equatorial bonds, thus they are comparatively longer than the equatorial bonds.
Question 6.
What do you mean by resonance? Explain with example.
Answer:
When properties of a molecule are hot explained by one structure and two or more than two structures are assigned to express its characteristics, it is said that molecule is resonance hybrid of these structures and this property is known as resonance.
Different resonating structures are exhibited by using sign (↔) in between these structures.
Resonance structures do not have any physical significance. Actually resonance theory is an imagination theory. As a result of resonance, energy of the compound decreases and stability increases. Energy of resonance hybrid is always less than the energy of various resonating structures.
Example: Carbon dioxide is normally represented by the following formula :
0 = C=0
On the basis of this structure bond length between O and C should be 1.22Å, but actual bond length is 1.15Å, which is in between the double and triple bond. Energy of formation of CO2 should be 300 kcal/mol but actual bond energy is 310 kcal/mol. Thus, there can be two formulae of CO2:
![]()
Other example of Resonance : Carbonate ion :
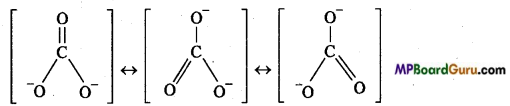
Conditions for resonance are as follows :
- Enthalpy of formation of all resonating forms is nearly same.
- In every formula, arrangement of atoms should be same.
- Number of unpaired electrons in all the resonating forms should be equal.
![]()
Question 7.
Explain the electrostatic attractive and repulsive force on the basis of formation of covalent bond. Or, What is Quantum theory of covalency for hydrogen molecule? Explain.
Answer:
During the formation of covalent bond, when two atoms come close to one another then two types of forces act between them:
- Attraction by nuclei of both the atoms to the electrons of the molecule.
- Repulsive force between the nuclei and the electrons of both the atoms.
When the resultant force of acting attractive force and repulsive force is attraction the energy of the system decrease and formation of bond is possible in this situation. But, if the resultant force of acting attractive force and repulsive force is repulsion, there is increase in energy and bond formation is not possible.
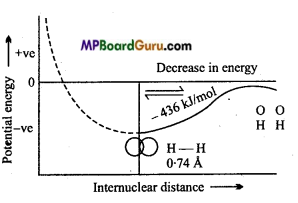
Example: Formation of H2 molecule :
If both the H-atoms are situated far from each other, then there is no attractive or repulsive force between them but when both the H-atoms are brought close to each other then the ahead to situations can be possible :
(i) When two H-atoms whose spin of electron is parallel to one another come closer then their potential energy continuously increases because strong repulsive force is present between both the electrons. Therefore this bond will be unstable.
(ii) If H-atoms of opposite spin come closer, strong attractive force between them leads to decrease in energy. Due to opposite spin electron density between both the nuclei increases which binds both the nuclei by strong electrostatic force. This bond is called covalent bond. If both the H-atoms are brought closer than the minimum distance, then repulsive force between nucleus-nucleus increases, by which energy increases continuously and bond again becomes unstable.
Question 8.
Structure of two molecules are given below :
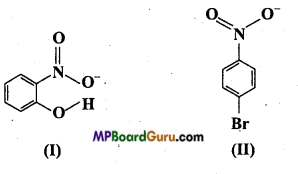
(i) Of the above in which compound intermolecular hydrogen bond and in which intramolecular hydrogen bond is present?
(ii) Melting point of a compound apart from other concepts is based on hydrogen bond. On this basis justify which of the above compound will have higher melting point?
(iii) Solubility of a compound depends upon its ability to form hydrogen bond. Which of the above compound will easily form hydrogen bond and will be more soluble in water?
Answer:
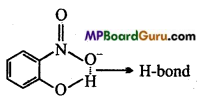
(i) Compound will form intramolecular hydrogen bond, when hydrogen atom in a molecule is present between two more electronegative atoms (O, F, N etc.) then intramolecular hydrogen bond is formed. In ortho nitrophenol (compound I), hydrogen atom is situated between two oxygen atom.
In compound (II) intermolecular hydrogen bond is formed. In p-nitro phenol vacant space is present between -NO2 and -OH group. Thus, hydrogen atom of one molecule forms intermolecular hydrogen bond with oxygen atom of other molecules.
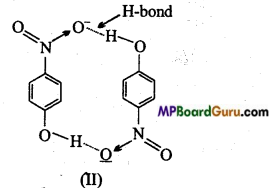
(ii) Melting point of compound(II) will be higher because its various molecules are associated with hydrogen bonds.
(iii) Due to intramolecular H-bond compound (I) cannot form H-bond with water, due to which it is less soluble in water. Whereas molecules of compound (II) can easily form H-bond with water due to which it is more soluble in water.
Question 9.
Explain the structure of the following molecules on the basis of VSEPR theory:
(a) CH4,(b) H2O,(c) NH3.
Answer:
(a) Structure of CH4: In methane the central carbon atom is surrounded by four electrons and it forms four similar C – H bonds. This molecule is of tetrahedral geometry due to sp3-hybridization. On the basis of maximum distance the paired electrons are at the four tips of the tetrahedron. Bond angle is 109°28′.
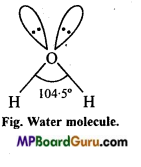
(b) Structure of H2O: In water molecule, central oxygen atom is also sp3-hybridized and structure is tetrahedral but oxygen atom has two lone pair of electrons. Thus, lp-lp and lp-bp repulsion exist. Due to this, bond angle reduces to 104°5′ in place of 109°28′ and shape becomes V shaped.
(c) Structure of NH3: In ammonia, central N atom is surrounded by four electron pairs, so shape of molecule is tetrahedral. According to VSEPR theory, the four groups around the central atom of ammonia should be tetrahedrally arranged at bond angle of 109°. But, the measured bond angle is 107°. This is explained on the basis of repulsive effect of the lone pair of electrons on bonding electrons. In ammonia molecule there is a lone pair of electrons on the N atom. Thus, the Shape of NH3 molecule is distorted and it looks like pyramidal and it is polar in nature.
Question 10.
Justify the following with reason :
(i) Covalent bonds are directional whereas ionic bond are non-directional.
(ii) Structure of water molecule is angular whereas carbon dioxide molecule is linear.
(iii) Ethyne molecule is linear.
Answer:
(i) Covalent bond, is formed by the overlapping of atomic orbitals. Direction of overlapping states the direction of bond. In ionic compounds, electrostatic area of ion is non-directional. On the basis of size of each cation is surrounded by anion in any direction. Similarly, anion is surrounded by cation. That is why, covalent bond is directional and ionic bond is non-directional.
(ii) In H2 ![]() oxygen is sp-hybridized with two lone pairs, sp3 hybrid orbitais are at the apices of the four tetrahedron. Two apices are surrounded by hydrogen atoms and remaining two apices are surrounded by lone pairs. Thus, due to lone pair, lone pair repulsive force bond angle decreases from 109-5° to 104-5° due to which shape of the molecule becomes V-shaped or bent or angular.
oxygen is sp-hybridized with two lone pairs, sp3 hybrid orbitais are at the apices of the four tetrahedron. Two apices are surrounded by hydrogen atoms and remaining two apices are surrounded by lone pairs. Thus, due to lone pair, lone pair repulsive force bond angle decreases from 109-5° to 104-5° due to which shape of the molecule becomes V-shaped or bent or angular.
In CO2 molecule, C-atom is sp-hybridized. Two hybrid orbitals are situated in opposite direction to each other forming an angle of 180°.
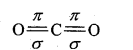
This is the reason that H2O molecule is angular whereas structure of CO2 is linear.
(iii) In Ethyne, both carbon atoms are sp -hybridized and the two orbitals (2px and 2py) are unhybridized. These two sp-hybrid orbitals lie in opposite directions making an angle of 180° due to which ethyne is linear.
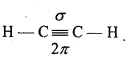
Question 11.
Write the main postulates of valence bond theory and state the limitations of this theory.
Answer:
Valence bond theory was proposed by Hitler and London which was modified by Pauling and Slater. The main postulates of this theory are as follows :
- According to this theory, covalent bond is formed by the partial overlapping of half filled orbitals between the atoms.
- Atomic orbitals which participate in overlapping possess electrons with opposite spin.
- Strength of bond depends upon the extent of overlapping.
- Directional strong bond is formed between two orbitals of similar stability, similar energy and symmetry.
- As a result of overlapping and pairing of electrons, energy is released and the system achieves a state of lower energy.
- Of both complete bond orbitals of each orbital is obtained. Therefore it is considered to be the property of both the atomic orbitals.
Limitations of Valence bond Theory :
- According to this theory, O2 molecule does not contain any unpaired electron. Thus, nature of O2 should be diamagnetic but O2 molecule is paramagnetic.
- Nothing is discussed about the formation of co-ordinate bond.
- This theory is unsuccessful in explaining the formation of ions like H2+ .
- It is unable to give any information regarding resonance.
![]()
Question 12.
Discuss the structure of PCl5 and SF6. Axial bonds in PCl5 is longer than equatorial bonds. Whereas in SF6 bond length of both axial and equatorial bonds are same discuss.
Answer:
Formation of PCl5:Electronic configuration of phosphorus is as follows :
15 P = 1s2,2s2 2p6,3s2,3p3

In the formation of PCl5 molecule, one s, three p and one rf-orbital are available for hybridisation. These orbitals combine to form five sp3d hybrid orbitals. These five hybrid orbitals are oriented towards the five comers of a trigonal bipyramid.

Again, in PCl5 three P – Cl bonds are situated on one plane and form an angle of 120°. These are known as equatorial bonds, remaining two P – Cl bonds lie above and below the equatorial bonds making an angle of 90°. These bonds are called axial bonds. Since axial bond feel more repulsion than equatorial bonds, thus they are comparatively longer than the equatorial bonds.
Formation of SF6.
Electronic configuration of S in ground state 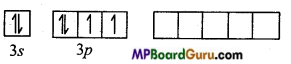
Electronic configuration of S in excited state 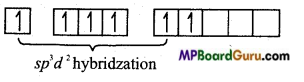
In SF6, sulphur is sp3d2 hybridized by which towards 6 corners octahedron the orbitals are orientated. These six sp3d2 hybrid orbitals overlap with p-orbitals of six fluorine atoms to form six similar S-F bonds.
Question 13.
What is bond order? What informations are given by bond order?
Answer:
Bond order is defined as half of the difference between the number of electrons in bonding molecular orbitals and anti-bonding molecular orbitals.
Bond order = \(\frac{1}{2}\) [No. of electron in bonding molecular orbitals – No. of electron in anti-bonding molecular orbital]
= \(\frac{1}{2}\) [Nb – Na].
Bond order in a molecule gives following informations :
- If bond order has positive value it indicates a stable molecule and if bond order has negative value or zero the molecule is unstable and is not formed.
- It tells about the number of covalent bonds in a molecule. Bond order of a molecule is equal to the number of covalent bond between the atoms in the molecule.
- Bond dissociation energy of a molecule is directly proportional to the bond order of the molecule. Greater the bond order, greater is the bond dissociation energy.
- Bond length is inversely proportional to the bond order of the molecule, That is greater the bond order, shorter will be the bond length.
- Paramagnetic molecules have fractional value of bond order while when bond order is a whole number, the molecule may or may not be paramagnetic.
Question 14.
Calculate bond order of O2, O2–, O22 and O2+.
Answer:
1. O2 [16] – 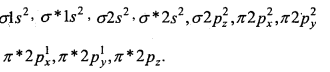
Bond order = \(\frac{1}{2}\) [10 – 6] = 2
2. O2–[17] – 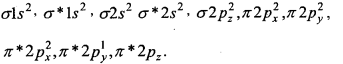
Bond order = \(\frac{1}{2}\) [10 – 7] = 1.5
3. O22- [18] – 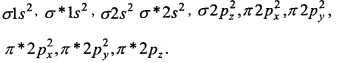
Bond order = \(\frac{1}{2}\) [10-8] = 1
4. O2+ [15] – 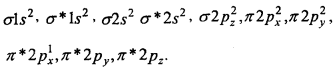
Bond order = \(\frac{1}{2}\) [10 — 5]
= 2.5.
Question 15.
Represent N2 molecular by figure on the basis of molecular energy level.
Answer:
Nitrogen molecule (N2): Each nitrogen atom contains seven electrons. Thus, total 14 electrons are filled in seven orbitals of increasing energy.
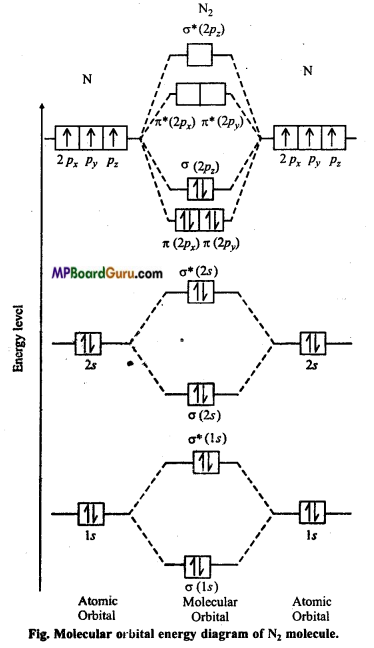
Molecular orbital structure of N2 molecule will be as follows :
N2: 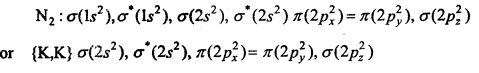
This structure shows that it contains 10 bonding and 4 anti-bonding electrons.
Bondorder =\(\frac{1}{2}\) (Nb-Na) = \(\frac{1}{2}\) (10-4) = 3.
Value of bond order is more. Hence value of bond energy should also be high. Experimental value of bond energy is 945 KJ mol-1 which proves the presence of paired electrons in nitrogen molecule. Thus, it is a diamagnetic molecule.
Question 16.
Represent by molecular energy level diagram that diatomic Neon molecule has no existence in nature.
Answer:
Electronic configuration of 10Ne = 1s2,2s2,2px22py22pz2.
Bondorder =\(\frac{1}{2}\) (Nb -Na) = \(\frac{1}{2}\) (10-10) = 0
Bond order zero means, no bond is formed between two Ne atoms. Thus, Ne2 (di¬atomic molecule) has no existence in nature.
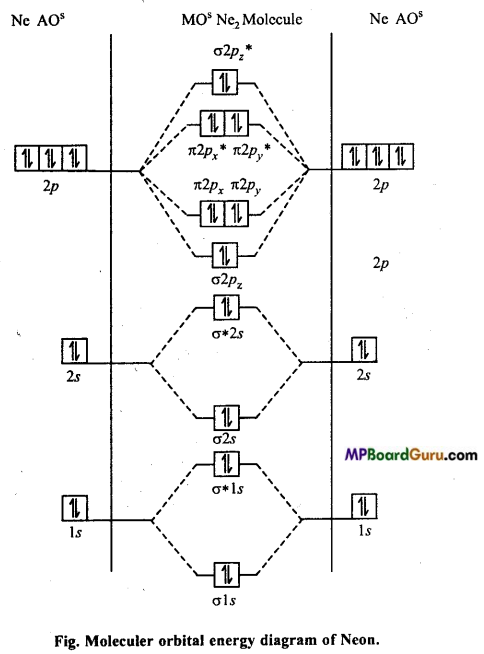
Question 17.
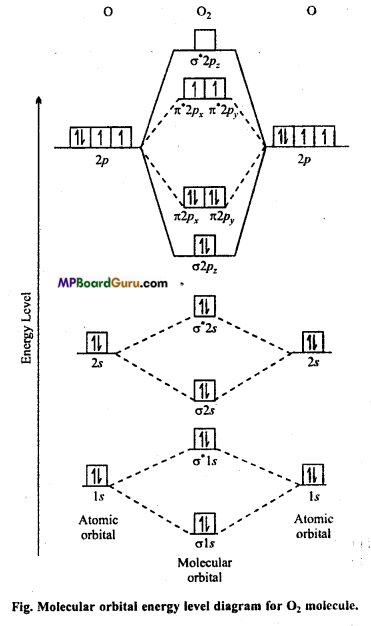
Draw molecular orbital diagram for O2 molecule.
Answer:
Oxygen molecule O2: Each oxygen atom has eight electrons. When two oxygen atom combines, molecular orbitals are formed. These molecular orbitals have following configuration.
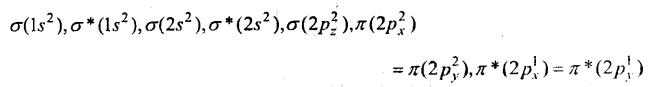
From the following configuration we have Nb = 10, Na = 6
∴ Bond order =\(\frac{1}{2}\) [Nb – Na] = [10-6] = 2
Hence, there is a double bond in oxygen molecule. Due to the presence of two unpaired electrons it is paramagnetic.
Its molecular orbital diagram is :
Chemical Bonding and Molecular Structure Class 11 Important Questions Objective Type
1. Choose the correct answer:
Question 1.
Which type of bond is formed between similar atoms :
(a) Ionic
(b) Covalent
(c) Co-ordinate
(d) Metallic.
Answer:
(b) Covalent
Question 2.
An atom form an anion :
(a) By loosing one or more electron
(b) By gaining one or two electrons
(c) By sharing electron pair
(d) None of these.
Answer:
(c) By sharing electron pair
Question 3.
Maximum number of hydrogen bonds formed by one water molecule in ice is :
(a) 4
(b) 3
(c) 2
(d) 1.
Answer:
(b) 3
Question 4.
Molecule with highest dipole moment is :
(a) CH4
(b) CHCl3
(c) CHI3
(d) CCl4.
Answer:
(b) CHCl3
![]()
Question 5.
Shape of ethylene molecule is :
(a) Tetrahedral
(b) Pyramidal
(c) Planar
(d) Linear.
Answer:
(b) Pyramidal
Question 6.
Ammonia molecule is formed due to hybridization of:
(a) dsp2
(b) sp3
(c) sp3d
(d) d2sp.
Answer:
(b) sp3
Question 7.
The high boiling point of water is due to :
(a) Weak dissociation of water molecules
(b) Hydrogen bonding among water molecules
(c) Its high specific heat
(d) Its high dielectric constant.
Answer:
(b) Hydrogen bonding among water molecules
Question 8.
Hydrogen bond is present in :
(a) HF
(b) HCl
(c) HBr –
(d) HI.
Answer:
(a) HF
![]()
Question 9.
Which of the following molecule has dipole moment zero :
(a) H2O
(b) CO2
(c) SO2
(d) NO2.
Answer:
(b) CO2
Question 10.
In which compound, sp2 hybrid orbitals are used :
(a) BCl3
(b) CH4
(c) NH3
(d) BeH2
Answer:
(a) BCl3
Question 11.
Which of the following molecules has linear structure :
(a) CO2
(b) H2O
(c) SO2
(d) H2O2.
Answer:
(a) CO2
Question 12.
Hybridization present in PCl5 molecule is :
(a) sp2d2
(b) sp3d
(c) spd3
(d) sp2d3.
Answer:
(b) sp3d
Question 13.
Number of electrons involved in the formation of bond in O2 :
(a) 2
(b) 4
(c) 6
(d) 10.
Answer:
(b) 4
Question 14.
In which molecule bond angle of central atom is maximum:
(a) NH3
(b) NH4
(c) PCl3
(d) SCl2.
Answer:
(b) NH4
Question 15.
H-bond is present in :
(a) CH4
(b) NaCl
(c) H2O
(d) CHCl3.
Answer:
(c) H2O
Question 16.
VSEPR (Valence Shell Electron Pair Repulsion) Theory was proposed by :
(a) Hund and Mullican
(b) Heisenberg
(c) Sizwick and Powel
(d) Heitler and London.
Answer:
(c) Sizwick and Powel
![]()
2. Fill in the blanks:
1. In CH4 ………………………… hybridization is found.
Answer:
sp3
2. Full name of LCAO is ………………………… .
Answer:
Linear combination of atomic orbital
3. Boiling point of NH3 is ………………………… than boiling point of PH3.
Answer:
Higher
4. ………………………… bond is formed by s-s overlapping.
Answer:
σ
5. H-O-H bond angle in water molecule is ………………………… .
Answer:
104.5°
6. ………………………… bond is formed due to p-p overlapping.
Answer:
π
7. σ bond is comparatively ………………………… than π -bond.
Answer:
Stronger
8. Geometry of molecule due to sp3 hybridization is ………………………… .
Answer:
Tetrahedral
9. With the formation of chemical bonds, there is a ………………………… in energy.
Answer:
Decrease
10. Diamond is a ………………………… of electricity but graphite is ………………………… of electricity.
Answer:
Bad conductor, good conductor
11. ………………………… hybridization is found in diambnd and graphite.
Answer:
sp2 and sp3
12. Hybridization in water molecule is ………………………… .
Answer:
sp3.
![]()
3. Match the following:
| ‘A’ | ‘B’ |
| 1. Shape of BeCl2 | (a) Square planar |
| 2. Shape of NH3 | (b) Angular |
| 3. Shape of diamond | (c) Linear |
| 4. Shape of water molecule | (d) Trigonal pyramidal |
| 5. Shape of [Ni(CN)4]2- | (e) Three-dimensional (planar tetrahedral). |
Answer:
1. (c) Linear
2. (d) Trigonal pyramidal
3. (e) Three-dimensional (planar tetrahedral)
4. (b) Angular
5. (a) Square planar.
4. Answer in one word/sentence:
1. What type of bond is present in nitrogen molecule?
Answer:
Triple covalent bond
2. Which type of bond is directional?
Answer:
Covalent bond
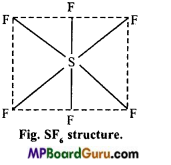
3: What is the shape of sulphur tetrafluoride?
Answer:
Square pyramidal
4. What type of chemical bond is found in sodium chloride?
Answer:
Electrovalent
5. Sugar is a covalent compound, yet it is soluble in water.
Answer:
Due to hydrogen bond
6. Bond angle of water molecule is.
Answer:
104.5°
7. Dipole moment of BF3 molecule is.
Answer:
Zero
8. Write the name of linear molecule.
Answer:
CO2
9. Which molecule show trigonal bipyramidal geometry?
Answer:
PCl5.