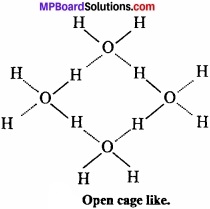
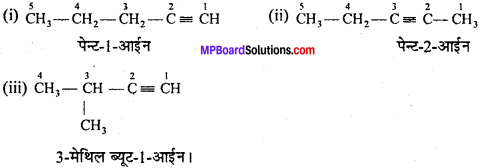
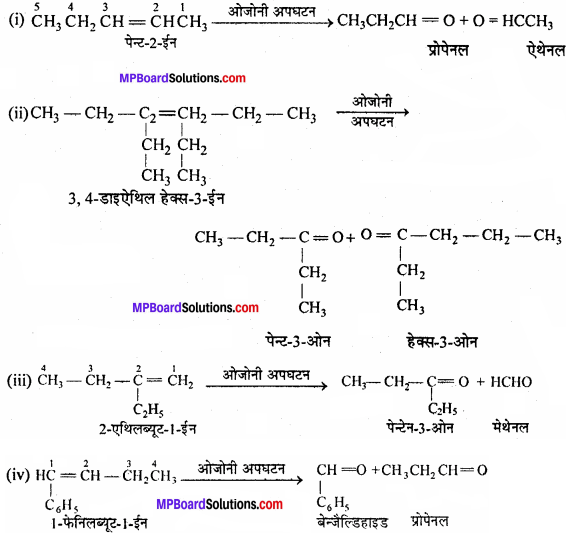
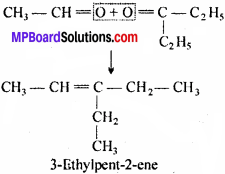
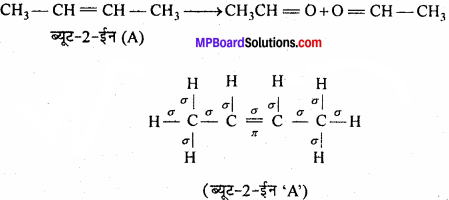
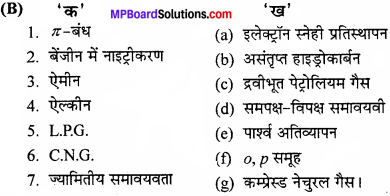
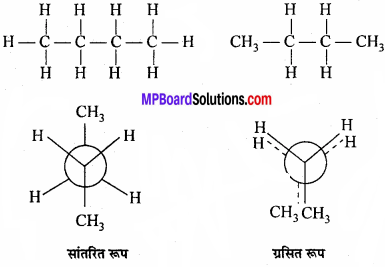
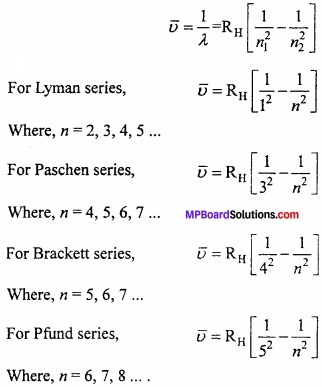
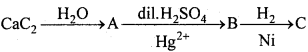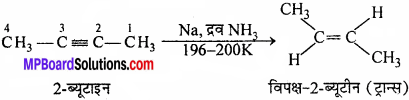MP Board Class 11th Chemistry Important Questions Chapter 10 s – Block Elements
s – Block Elements Important Questions
s – Block Elements Objective Type Questions
Question 1.
Choose the correct answer:
Question 1.
Plaster of Paris is :
(a) (CaSO4)2.H2O
(b) CaSO4.2H2O
(c) CaSO4.H2O
(d) CaSO4
Answer:
(a) (CaSO4)2.H2O
Question 2.
Lowest melting point compound:
(a) LiCl
(b)NaCl
(c) KCl
(d) RbCl
Answer:
(a) LiCl
Question 3.
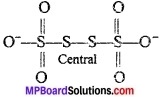
Active constituent of Bleaching powder:
(a) CaOCl2
(b) Ca(OCl)Cl
(c) Ca(O2Cl2)
(d) CaCl2O2
Answer:
(b) Ca(OCl)Cl
![]()
Question 4.
Hydration energy of Mg2+ ion will be more than:
(a) Al3+
(b) Na+
(c) Be2+
(d) Mg3+
Answer:
(b) Na+
Question 5.
Which magnetic property is present in alkaline earth metals:
(a) Diamagnetic
(b) Paramagnetic
(c) Ferro – magnetic
(d) Anti – magnetic
Answer:
(a) Diamagnetic
Question 6.
Solubility of which sulphate is the least:
(a) BaSO4
(b) MgSO4
(c) SrSO4
(d) CaSO4
Answer:
(a) BaSO4
Question 7.
Compounds of which element are mainly covalent:
(a) Ba
(b) Sr
(c) Ca
(d) Be
Answer:
(d) Be
![]()
Question 8.
Important ore of magnesium is:
(a) Malachite
(b) Kaesiterite
(c) Camallite
(d) Galena.
Answer:
(c) Camallite
Question 2.
Fill in the blanks:
- Radius of Ca2+ ion is less than K+ because of ……………………………
- On heating Rb(ICl2) decomposes to form ………………………….. and ………………………….
- Be(OH)2 is soluble in both acid and base because it is of ……………………… nature.
- Lithium resembles ………………………… element of group 2.
- Sodium metal gives blue colour in liquid ammonial this is because of ………………………………
- Potassium forms three oxides ………………………. and …………………………..
Answer:
- High positive charge
- RbCl, ICI
- Amphoteric
- Mg
- e–(NH3)2
- K2O, K2O2, KO2
![]()
Question 3.
Answer in one word/sentence:
- Sodium metals are kept in kerosene oil. Why?
- What is lithopone?
- Which element is present in teeth and bones?
- What is Caustic soda?
- What is Sorrel cement?
- Write formula of Plaster of Paris?
Answer:
- Highly reactive
- BaSO4 and ZnS
- Calcium
- NaOH
- Mixture of MgCl2 and MgO
- (CaSO4)2H2O
s – Block Elements Very Short Answer Type Questions
Question 1.
Arrange the alkali metals in the increasing order of their reactivity?
Answer:
The reactivity of alkali metals increases from top to bottom.
Be < Mg < Ca < Sr < Ba < Ra.
Question 2.
Write the name of two ores of lithium with formula?
Answer:
Ores of lithium are:
- Spodumene LiAl(SiO3)2
- Lepidolite Li2Al2(SiO3)3.F(OH)2.
Question 3.
Write the name of two ores of magnesium?
Answer:
- Camolite
- Dolomite.
Question 4.
Why alkali metals are kept in kerosene oil?
Answer:
Due to high reactivity.
![]()
Question 5.
What is the formula of global salt?
Answer:
Na2SO4.10H2O.
Question 6.
What is Lithophone?
Answer:
BaSO4 + ZnS.
Question 7.
What is the formula of hydrolite?
Answer:
CaH2.
Question 8.
Carnolite is ore of which metal?
Answer:
Magnesium (Mg).
Question 9.
Which alkali metal show radioactivity?
Answer:
Radium.
Question 10.
Which compound is useful in purification of air in aircrafts?
Answer:
Potassium superoxide.
![]()
Question 11.
Name one mineral in which Ca and Mg both are present?
Answer:
Name: Dolomite, Formula: MgCO3.CaCO3.
Question 12.
Which flux is used for the removal of acidic impurities in metallic processes?
Answer:
- Limestone CaCO3
- Magnesite MgCO3.
Question 13.
Formula of Nitrolium is?
Answer:
CaCN2 and C.
Question 14.
Which forms curtain of smoke?
Answer:
SiCl4.
![]()
Question 15.
What is used to join the fractured bone and make statues?
Answer:
Plaster of paris.
Question 16.
What is the order of stability of carbonates of alkali metals?
Answer:
BeCO3 < MgCO3 < CaCO3 < SrCO3.
Question 17.
Write the configuration of alkali metals?
Answer:
ns1-2.
Question 18.
What is the formation of Apsum and Gypsum salt?
Answer:
Magnesium sulphate (MgSO4.2H2O) and Calcium sulphate (CaSO4.2H2O).
s – Block Elements Short Answer Type Questions – I
Question 1.
Why alkali metal do not found in free state?
Answer:
Alkali metals are highly reactive and electropositive because the value of ionization energy is very low due to larger size thus alkali metals easily donate electron and form positive ion. (MPBoardSolutions.com) These positive ion easily combine with oxygen, moisture and other electronegative element present in nature and form ionic compound.
Question 2.
Explain, why sodium is less reactive than potassium?
Answer:
The ionization enthalpy of sodium is higher than that of potassium. Therefore, sodium lose electron less readily as compared to potassium. Hence, sodium is less reactive than potassium.
Question 3.
Potassium carbonate cannot be prepared by Solvay process. Why?
Answer:
Potassium carbonate cannot be prepared by Solvay process because potassium bicarbonate being more soluble than sodium bicarbonate and does not get precipitated when CO2 is passed through a concentrated solution of KCl with NH3.
![]()
Question 4.
Why is Li2CO3 decomposed at a lower temperature whereas Na2CO3 at higher temperature?
Answer:
Lithium is less electropositive than sodium and therefore, carbonates of lithium is less stable than that of sodium. Li2CO3 is not so stable to heat and therefore, decomposes at lower temperature. (MPBoardSolutions.com) This is because lithium being veiy small in size polarizes a large. CO32- ion leading to the formation of Li2O and CO2. On the other hand, Na2CO3 is very stable and decomposes at higher.
Question 5.
I – A and II – A group is called s – block elements. Why?
Answer:
I – A and II – A group elements have their electronic configuration of ns1 and ns2. So the last electron fill in 5 – block so the I – A and II – A group is called 5 – block elements.
Question 6.
What is Soral cement and write its uses?
Answer:
When MgCl2 solution reacts with MgO and product is formed as MgCl2.2MgO. nH2O. It is a white paste and called as Soral cement.
Uses:
- It is used to fill the teeth cavity.
- They use in porcelain in point.
Question 7.
Find the oxidation state (O.S.) of Na in Na2O2?
Answer:
Let the O.S. of Na in Na2O2 = x
One peroxide bond (Na – O – O) is present in Na2O2 where the O.S. of O = -1
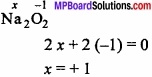
So, in Na2O2 the O.S. of Na = +1.
Question 8.
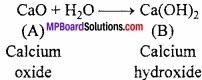
How calcium sulphate is prepared? Write its uses?
Answer:
In the lab, calcium sulphate is formed by Ca oxide, carbonate, chloride. They react with dil. H2SO4 to form calcium sulphate.
CaO + H2SO4 → CaSO4 + H2
Ca(OH)2 + H2SO4 → CaSO4 + 2H2O
CaCl2 + H2SO4 → CaSO4 + 2HCl
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
Question 9.
What is Gypsum? How plaster of Paris is formed by gypsum?
Answer:
Calcium sulphate CaSO4.2H2O is called gypsum. Gypsum is heated at 120° – 130°C and 3 parts of water molecule are released and plaster of Paris is formed.
![]()
The plaster of Paris again absorb the water molecule and again get converted to calcium sulphate (Gypsum).
Question 10.
Why during the formation of quick lime the temperature of furnance is not kept more than 1000°C? Explain with equation?
Answer:
During the formation of quick, lime the temperature is maintained at 1000°C because at high temperature the clay present as impurity in the limestone combines with lime producing fusible silicate which fill the pores of lime. Due to this reason slaking of lime become very difficult.
1000°C.
CaO + SiO2 \(\underrightarrow { 1000^{ \circ }C } \) CaSiO3.
![]()
Question 11.
Why sodium kept in kerosene oil?
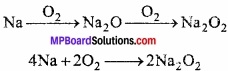
Answer:
Alkali metals are very reactive specially against electronegative elements like oxygen, moisture and CO2. These are oxidized quickly from oxide and hydroxide due to their reactivity. Sodium is kept in kerosene oil to prevent oxidation on exposure to air.
4Na + O2 → 2Na2O
2Na + H2O → Na2O + H2
Na2O + H2O → 2NaOH.
Question 12.
Write the formula of lime water. What happens when CO2 passes through it?
Answer:
The formula of lime water is Ca(OH)2. Due to the flow of the CO2 gas in Ca(OH)2 it becomes milky due to formation of a white precipitate of calcium carbonate. (MPBoardSolutions.com) On passing excess of CO2, milkyness disappears and calcium bicarbonate is formed which is soluble in water.
Ca(OH)2 + CO2 → CaCO3 + H2O
CaCO3 + CO2 + H2O → Ca(HCO3)2.
Question 13.
Why K2CO3 is not prepared by Solvay method?
Answer:
Potassium carbonate cannot be prepared by Solvay method because the potassium salt analogous to NaHCO3 is KHCO3 which is much soluble and hence, cannot be obtained by crystallization.
Question 14.
Which metal is used in photochemical cell and why?
Answer:
In photochemical cell potassium and caesium metals are used, as their ionization energy is very low.
Question 15.
Why the extraction of sodium from sodium chloride cannot be done by general reducing agents?
Answer:
Sodium is a strong reducing agent. In electrochemical series, it is present at top position. Due to new availability of strong reducing agent than sodium, it cannot be reduced by normal reducing agents. It can only be reduced by electrolytes.
![]()
Question 16.
Why Li and Be have the tendency to form covalent compounds? Explain?
Answer:
Due to small size of Li and Be atoms and their high ionization energy, the electrons of the valence shell are firmly bound to their nuclei. The polarizing power of their ions is also high due to high charge density, hence Li and Be form covalent compounds.
Question 17.
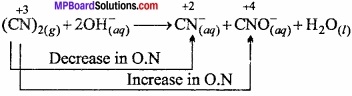
Write the Lewis structure of O–2 ion and write the O.N. of each O atom. In this ion what is the average oxidation state of O?
Answer:
Lewis structure of O–2 ion = ![]()
Oxygen without charge have 6 electrons. So, its O.S. = 0, but oxygen with -1 oxidation state have 7 electrons so its O.S. = —1.
Oxidation state of each oxygen atom = \(\frac{-1}{2}\)
O2– = 2x = -1
x = \(\frac{-1}{2}\)
Question 18.
In Solvay’s process, can be obtain sodium carbonate by direct reaction of ammonium carbonate and sodium chloride?
Answer:
No, because the reaction between ammonium carbonate and NaCl.
(NH4)2CO3 + 2NaCl ⇄ Na2CO3 + 2NH4Cl
As the products obtained are highly soluble the equilibrium will not shift to,yards forward direction. This is the reason why NaCO3 cannot be prepared by reaction of (NH4)2CO3 and NaCl in Solvay’s process.
![]()
Question 19.
AH compounds of alkali metals are easily soluble in water but lithium compounds are more soluble in organic solvents? Explain?
Answer:
The size of LT ion is small and thus, it has high polarizing power. This brings covalent character in lithium compounds. Due to covalent character, Li compounds are soluble in organic solvents.
Question 20.
Why are potassium and caesium rather than lithium used n photoelectric cells?
Answer:
Potassium and caesium have much lower ionization enthalpy than that of lithium. Therefore, these metals on exposure to light, easily emit elec arm but lithium does nN. Therefore, K and Cs rather than Li are used in photoelectric cells.
Question 21.
Beryllium chloride (BeCl2) produces smoke when kept in air. Why?
Answer:
Normally air contains moisture, thus beryllium halide hydrolyses in water and releases HCl due to which it produces smoke in air.
BeCl2 + 2H4O → Be(OH)2 + 2HCl.
Question 22.
On moving downward in the first group the hardness of the elements increases. Why?
Answer:
In the first group on moving downwards along with the increase in size cf the elements their density also increases and the force of attraction between its atoms increases due to which there is an increase in their hardness.
s – Block Elements Short Answer Type Questions – II
Question 1.
Why s – block and p – block elements are called representative elements?
Answer:
The elements present in the s and p-block are called normal or representative elements.
- The elements of group 1 and 2 constitute the s – block of the periodic table. These are known as s – block elements because the last electron in them enters the s – orbital of the valence shell.
- The elements of p group of the periodic table these are known as p – block elements because the last electron in them enters the p-orbital of the valence shell.
Question 2.
Why alkaline metals are strong or reducing agents?
Answer:
Due to less ionization energy of alkali metals. They have tendency to loose electron (Get oxidized) and form positive ion (M+) also alkali metals have negative value of standard reduction potential. (MPBoardSolutions.com) Therefore, alkali metals are good reducing agent.
![]()
Question 3.
Why are BeSO4 and MgSO4 readily soluble in water while CaSO4, SrSO2 and BaSO4 are insoluble?
Answer:
The hydration energy of SO4 of alkaline earth metals decreases down the group. The high hydration energy of Be2+ and Mg2+ diminishes the lattice energy due to this their SO2 are soluble in water. But in case of Ca2+, Sr2+ and Ba2+ the hydration energy is low due to this the SO4 are insoluble in water.
Question 4.
Why the reducing power of lithium is high in solution?
Answer:
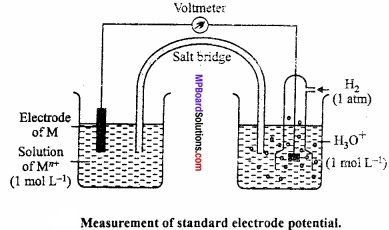
Electrode potential is a measure of the tendency of an element to loose electron in aqueous solution. It may depend on the following three factors:

With the small size of its ion, Li has the highest hydration enthalpy. However, ionization enthalpy of Li is highest among alkali metals but hydration (MPBoardSolutions.com) enthalpy predominates over I.P. Therefore, Li is the strongest reducing agent in aqueous solution.
Question 5.
Explain, why can alkali and alkaline earth metals not be obtained by chemical reduction method?
Answer:
The s – block elements themselves are good reducing agents therefore, reducing agent better than s – block elements are not available. Therefore, the chlorides, oxides etc. of s – block elements cannot be reduced to obtain metal.
Question 6.
Explain why alkaline metals form M+ cation not M+2 type of cation?
Answer:
There is one electron in the valence shell of alkali metals. The ionization energy is very low due to bigger size. So they can easily donate electron and can form M+ cation. (MPBoardSolutions.com) In the M+ state the electronic configuration becomes similar to nobel gases and stable so in this state they become unreactive and the ionization energy become high. So they did not form M2+ ion.
Question 7.
In alkali metals, which metal is strongest reducing agent. Why?
Answer:
The reducing character increases from sodium to caesium. However, lithium is the strongest reducing agent among all the alkali metals. Inspite of its highest I.P. This is because of extensive hydration of Li+ ions and large amount of energy released during hydration more than compensates the higher I.P. value of Lithium.
![]()
Question 8.
Why alkali metals are not found in free state in nature? Or, Why alkali metals always form ionic compounds?
Answer:
Due to bigger size of alkali metals, the ionization energy is very low. So they can easily donate electron and form positive ion. So due to positively charge and highly reactive nature they combine with the (MPBoardSolutions.com) electronegative elements in the nature like moisture, CO2 etc. and form ionic compounds. Therefore, they cannot be found in free state in nature.
Question 9.
Why alkali metal give flame test?
Answer:
The ionization energy of alkali metal is very low. When these elements or their compounds are heated in Bunsen flame electron present in valency shell absorb energy and easily goes to higher energy level. When these excited electron comes to ground state they emit energy in the form of radiation in visible region and give characteristic colour to flame.
Question 10.
Why Be and Mg do not give flame test?
Answer:
Beryllium and Magnesium atoms in comparison to other alkaline earth metals are comparatively smaller and their ionization energy are very high. (MPBoardSolutions.com) Hence, the energy of the flame is not sufficient to excite their electrons to higher energy levels. These elements therefore do not give any colour in Bunsen flame temperature.
Question 11.
When an alkali metal dissolves in liquid ammonia, the solution acquires different colours? Explain the reason for this type colour change?
Answer:
All alkali metals dissolve in liquid ammonia giving highly conducting deep blue solutions.
M + (x + y )NH3 M+ (NH3) → x + e– (NH3 )y
When ordinary light falls on these ammoniated electrons. They get excited and jump to higher energy levels by absorbing energy corresponding to red region of the visible light. (MPBoardSolutions.com) As a result transmitted light is blue which imparts blue colour to the solution. However, when the concentration increases the ammoniated metal ion may get bound by free electrons and colour becomes copper bronze.
![]()
Question 12.
Why Be and Mg do not give flame test but other metals give? Why?
Answer:
Except beryllium and magnesium all the alkaline earth metals impart characteristic colours to Bunsen flame. Due to small size of Be and Mg atom, the energy required to excite the valency electrons is very high which is not obtained in Bunsen flame. That is why Be and Mg do not impart any colour to flame.
Question 13.
Give two uses of each:
- Caustic soda
- Sodium carbonate
- Quick lime.
Answer:
1. Uses of Caustic soda:
- In soap and paper industry.
- For mercerization of cotton thread.
2. Uses of Sodium carbonate:
- Washing soda is used for washing clothes.
- Removes permanent hardness of water.
3. Uses of Quick lime:
- For making statues, floor, buildings in the form of marble.
- For manufacture of lime, cement, glass and washing soda.
Question 14.
Alkali metals form blue coloured solution when dissolved in ammonia, which is a strong electrolyte. Give reason with equation?
Answer:
Alkali metals dissolve in liquor ammonia to form deep blue coloured solution of high electrical conductivity.
M + (x+y) NH3 → [M(NH3)x] + [e(NH3)y]–
The blue colour of the solution is due to ammoniated electrons positive ion and electrons are responsible for the conductivity.
Question 15.
Na is alkaline or Na2O? Clarify the statement?
Answer:
Monoxide of all alkali metals are alkaline and form strongly alkaline solution in water.
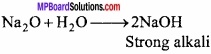
Na2O is alkaline because it reacts with water to form NaOH. Na also react with water to form NaOH but it first forms Na2O and then NaOH, thus Na2O us alkaline not Na.
4Na + 2H2O → 2Na2O + 2H2
Na2O + H2O → 2NaOH
![]()
Question 16.
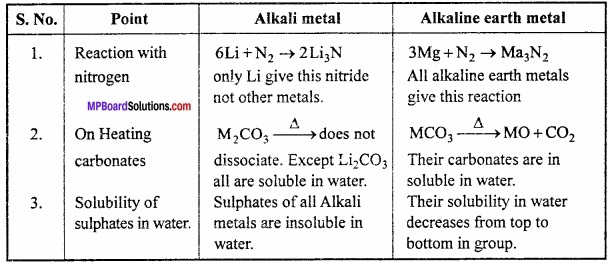
Compare Alkali metals and Alkaline earth metals on the basis of following points:
- Reaction of heat on carbonate
- Reaction with nitrogen
- Solubility of sulphates in water.
Answer:
Comparison of Alkali metals and Alkaline earth metals:
Question 17.
Hydroxides and Carbonates of (Sodium and Potassium) Alkali metals are completely soluble in water whereas that of (Magnesium and Calcium) Alkaline earth metals are partially soluble?
Answer:
All alkali metal carbonates are soluble in water because the value of their Lattice energy is less than their hydration energy.
Lattice energy < Hydration energy (Compound soluble)
Alkaline earth metal carbonates are insoluble because the value of their Lattice energy is more than their hydration energy.
Lattice energy < Hydration energy (Compound insoluble)
∆Hsolution = ∆HHydrogen energy + ∆HLattice energy
Question 18.
Why are lithium salts commonly hydrated and those of the other alkali ions usually anhydrous?
Answer:
Because of smallest size among alkali metals, Li+ can polarize water molecule more easily than the other alkali metal ions and hence get attached to lithium salts as water of crystallization.
Question 19.
Why is LiF almost soluble in water whereas LiCI soluble not only in water but also in acetone?
Answer:
To make compound water soluble, its lattice energy should be low and hydration energy should be high. Lattice energy of LiCl is less than that of LiF. The difference in these two energies for LiCl and LiF is 31 and 14 kJ/mol respectively. (MPBoardSolutions.com) The difference is larger for LiCl, which is soluble in water. Moreover, lattice energy of LiF is much higher than that of LiCl. It makes LiF sparingly soluble in water. Moreover, LiCl is largely covalent, so it is soluble in organic solvent such as acetone.
Question 20.
If the alkali metals are kept open in the air, after sometime the metallic brightness is lost. Why?
Answer:
On exposure to moist air alkali metals soon get covered with a thick crust of their oxide, hydroxides and carbonate hence, their surface get tarnished. For this reason these metals are stored under kerosene and which prevents them from coming in contact with air and moisture.
4M + O2 → 2M2O
M2O + H2O → 2MOH
2MOH + CO2 → M2CO3 + H2O
Here, M is any alkali metal.
![]()
Question 21.
Among LiCl and RbCl which will ionize more. Why?
Answer:
Among LiCI and RbCl, RbCl is more reactive because LiCl is slightly covalent in nature due to which it is soluble in organic solvent like pyridine and alcohol.
Question 22.
Among alkali metals and alkaline earth metals whose carbonates are soluble in water? Or, The carbonates of alkali metals are souble in water but that of alkaline metals are insoluble?
Answer:
Carbonates of alkali metals are soluble in water because its hydration energy is greater than lattice energy. Whereas alkaline earth metals are smaller in size and have high density, so its hydration energy is lower than lattice energy. So the carbonates of alkaline earth metals are insoluble in water.
Question 23.
Give the differences between the 8eCl2 and other chlorides of alkaline earth metals?
Answer:
1. Anhydrous halide are deliquescent and they absorb moisture or water forming hydrated salt.
Example: MgCl2.6H2O, CaCl2.6H2O, BaCl2.2H2O, etc.
2. BeCl2 fumes on hydrolysis in moist air.
BeCl2 + 2H2O → Be(OH)2 + 2HCl
3. BeCl2 has different structure in solid and in vapour state. In solid state, it exists as polymeric chain in which each Be atom is surrounded by four chlorine atom. Two of the chlorine atom are covalently bonded while, other two are bonded through co – ordinate bond. In vapour state, at a temperature above 1200 K it has linear monomeric structure with zero dipole moment. Below 1200 K it exist as a dimer.
4. Except BeCl2 and MgCl2 other metal chloride give characteristic colour to the flame.

5. Anhydrous CaCl2 has strong affinity for water therefore, it is used as dehydrating agent.
Question 24.
Why solubility of BaSO4 is less than CaSO4?
Answer:
The solubility of sulphates decreases from top to bottom as lattice energy is same. But as we move top to bottom the atomic size increases and hydration energy decreases so solubility decreases.
Question 25.
Why ionization energy of Be is more than B?

These orbitals of Be is full – filled so this element is stable but orbitals of B is not full – filled (Completely), so this element is not stable.
Question 26.
How sodium carbonate is formed by soda process? Write its principle?
Answer:
Solvay method or Ammonia soda process: It has replaced Le – Blanck process and is most commonly employed.
Principle:
In this process, concentrated solution of sodium chloride (Brine) is saturated with NH3 to form ammoniacal sodium chloride. On passing CO2 gas to this solution, ammonium bicarbonate is formed which reacts with sodium chloride and forms sodium bicarbonate.
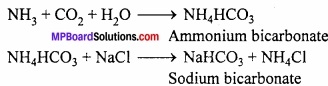
Question 27.
How will you prepare
- Sodium bicarbonate
- Sodium hydroxide
- Sodium silicate from sodium carbonate?
Answer:
1. In sodium carbonate (Aqueous solution) pass CO2 gas and sodium bicarbonate is formed (White precipitate).
Na2CO3 + H2O + CO2 → 2NaHCO3
2. Sodium carbonate is boiled with limewater then sodium hydroxide is formed.
Na2CO3 + Ca(OH)2 → 2Na0H + CaCO3
3. Sodium carbonate is treated with silica to form sodium silicate.
![]()
Question 28.
What is Baking soda? Write its method of preparation, properties and uses?
Answer:
Sodium Bicarbonate (NaHCO3):
It is also known as sodium hydrogen carbonate or baking soda.
Method of preparation:
Sodium bicarbonate is obtained as an intermediate in the manufacture of sodium carbonate by Solvay’s process. It can be also obtained by passing carbon dioxide through aqueous solution of sodium carbonate.
Na2CO3 + H2O + CO2 → 2NaHCO3
Physical property:
It is a white, crystalline solid, slightly soluble in water.
Chemical properties:
1. Effect of heat:
When heated to 373 K it decomposes into Na2CO3 and CO2 is set free.
![]()
2. Action of water:
Sodium bicarbonate hydrolyses when dissolved in water. Its aqueous solution is alkaline.
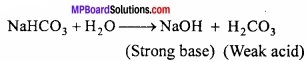
Uses:
- As a source of carbon dioxide in fire extinguisher.
- It acts as mild antiseptic for skin infection.
- As a component of baking powder.
- As a digestive powder to remove acidity of stomach.
Question 29.
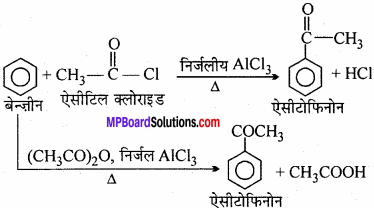
Describe the Le – Blanck method of preparation of sodium carbonate with chemical equation? How Solvay method is better than this?
Answer:
Le – BIanck method:
Sodium chloride when heated with cone. H2SO4 produces sulphates and HCl gas. Mixture of sodium sulphate, calcium carbonate and coke on heating provide sodium carbonate and calcium sulphide. This mixture is called blanck ash. (MPBoardSolutions.com) Now in this mixture water is added and filtered. Gas is removed as residue while sodium carbonate being soluble goes into filtrate. Evaporation of filtrate provide solid sodium carbonate.
2NaCl + H2SO4 → Na2SO4 + 2HCl
Na2SO4 + 4C → Na2S + 4CO
Na2S + CaCO3 → Na2CO3 + CaS
Advantages of Solvay method:
- It is cheap
- Pure Na2CO3 is formed
- No harmful smoke is obtained
- In the middle of reaction NaHCO3 is formed which is a useful compound.
s – Block Elements Long Answer Type Questions – I
Question 1.
Describe the manufacture of caustic soda by Nelson cell on the following point: Labelled diagram, Chemical reactions.
Answer:
It consists of perforated steel tube Graphite anode lined inside with asbestos act as cathode. It is fitted with NaCl solution (brine) and is suspended in the steel tank. Here asbestos lining separates cathode from anode.
One passing current electrolysis of NaCl takes place. (MPBoardSolutions.com) Chlorine is liberated at anode and escapes out. Sodium ions passed through asbestos and is liberated at cathode they reacts with steam for caustic soda.
![]()
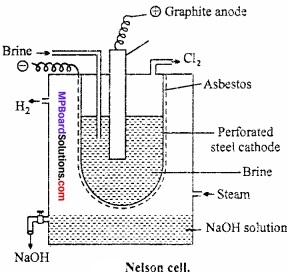
At anode:
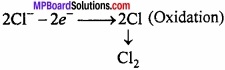
At cathode:
2Na+ + 2e– → 2Na (Reduction)
2Na + 2H2O → 2NaOH + H2
Question 2.
Ions of an element of group 1 participate in the transmission of nerve signals and transport of sugars and amino acids into ceils. This element imparts yellow colour to the flame in flame test and forms an oxide and a peroxide with oxygen. Identify the element and write chemical reaction to show the formation of its peroxide. Why does the element impart colour to the flame?
Answer:
The element is sodium. This imparts yellow colour to the flame. Na+ ions participate in the transmission of nerve signals and transport of sugars and amino acids to cell. It forms a monoxide (Na2O) and a peroxide (Na2O2).
The reason for flame colouration is, ionisation energy of Na is low. (MPBoardSolutions.com) Therefore, when Na metal or its salts is heated in Bunsen flame, its valence shell electron is excited to higher energy levels by absorption of energy. When the excited electron returns to the ground state, it emits extra energy in the yellow region of electromagnetic spectrum. Therefore, Na imparts yellow colour to the flame.
![]()
Question 3.
Write the diagonal relationship between Be and Al?
Answer:
Diagonal relationship between Beryllium and Aluminium:
Beryllium resembles aluminium of group 3 in the following properties:
- Both beryllium and aluminium form covalent compounds.
- Both metals are passive towards reaction with concentrated HN03, because they are covered with a layer of oxide on their surface.
- Both the metals are of weak electropositive nature.
- Both do not form hydride quickly.
- Carbides of both the metals react with water to form methane.
Be2C + 2H2O → 2BeO + CH4
Al4C3 + 6H2O → 2AlCl3 + 3CH4
6. Oxides of both are soluble in water and form hydroxides which are amphoteric in nature and react with acid and base to form salt.
BeO + 2HCl → BeCl2 + H2O
Al2O3 + 6HCl → 2AlCl3 + 3H2O
BeO + 2NaOH → Na2BeO2 + H2O
Al2O3 + 2NaOH → 2NaAlO2 + H2O
7. Both Be and Al do not give colour to the flame.
Question 4.
When water is added to compound (A) of calcium, solution of compound (B) is formed. When carbon dioxide is passed into the solution, it turns milky due to the formation of compound (C). (MPBoardSolutions.com) If excess of carbon dioxide is passed into the solution milkiness disappears due to the formation of compound (D). Identify the compounds A, B, C and D. Explain why the milkyness disappears in the last step?
Answer:
The compound (A) is quick lime, CaO. This combines with water and forms calcium hydroxides Ca(OH)2.
When CO2 is passed through the solution having Ca(OH)2, the solution turns milky due to formation of calcium carbonate, CaCO3.

When excess of CO2 is passed into milky solution the milkiness disappears due to formation of calcium bicarbonate Ca(HCO3)2 which is soluble.

Question 5.
How is lithium different from other members of its group?
Answer:
Anomalous behaviour of Lithium:
Properties of lithium are different than other members of the group due to the following reasons:
- Size of lithium atom and ion is very small.
- Due to small size, polarizing power of lithium ion is high, due to w hich compounds possess covalent character.
- In comparison to other alkali metals its electropositive nature is less and ionization energy is high.
Lithium differ from alkali metals of group I due to following properties:
- Lithium is comparatively harder than the other alkali metals.
- Melting and boiling point of lithium is comparatively high.
- Lithium reacts with oxygen to form only normal oxide (Li2G), whereas other metals form peroxide (M2O2) and superoxide (MO2).
- Lithium hydride (LiH) is more stable as compared to hydrides of other metals.
- Lithium hydroxide (LiOH) is a weak base and is partially soluble in water, but hydroxides of other metals of the group are more soluble in water.
- Lithium forms nitride (Li3N) with nitrogen, whereas other metals of the group do not form nitride.
- Lithium nitrate when heated decomposes to nitrogen dioxide and oxygen.
4LiNO3 \(\underrightarrow { \Delta } \) 2Li2O + 4NO2 + O2
Whereas sodium nitrate and potassium nitrate when heated strongly form corresponding nitrate with the release of oxygen.
2NaNO3 \(\underrightarrow { \Delta } \) 2NaNO2 + O2
Question 6.
What happens when:
- Magnesium is burnt in air –
- Quick lime is heated with silica –
- Chlorine reacts with slaked lime –
- Calcium nitrate is heated?
Answer:
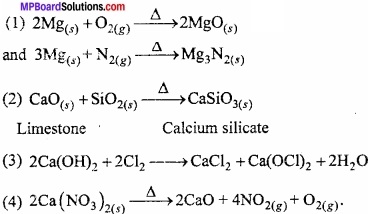
s – Block Elements Long Answer Type Questions – II
Question 1.
How is sodium carbonate manufactured by Solvay process?
Answer:
Principle:
In this process, first concentrated solution of sodium chloride (Brine) is saturated with NH3 to form ammanica sodium chloride.(MPBoardSolutions.com) On passing CO2 gas to this, ammonium bicarbonate is formed which reacts with sodium chloride and forms sodium bicarbonate. Precipitate of sodium bicarbonate is filter ed which on calcination gives sodium carbonate.
NH3 + CO2 + H2O → NH4HCO3
NH4CO3 + 2NaCl → NaHCO3 + NH4Cl
Na2HCO3 → Na2CO3 + H2O + CO2
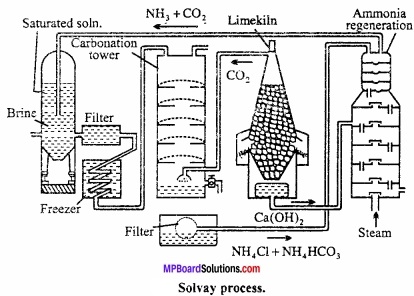
CO2 formed is used agiain for carbonation NH4Cl formed on treatment with slaked lime gives NH3 which is used for saturation of brine.
2NH4CL + Ca(OH)2 → CaCl2 + 2H2O + 2NH3
The various steps and reactions involved are given below:
1. Saturating tank:
In this tank brine (NaCl) is saturated with ammonia. NaCl solution is introduced from the top while ammonia is introduced from bottom so that ammonium brine is formed and which collects at bottom. Calcium and magnesium salts are present as impurity in sodium chloride solution. They get precipitated as hydroxides by ammonium hydroxide.
2. Filter:
Ammonical brine is filtered to removed the precipitated hydroxides of calcium and magnesium.
3. Cooler:
The filtrate is cooled by passing through condenser. Cooling is necessary because when ammonia dissolves in brine a lot of heat is produced.
4. Carbonating tower:
The cold solution of brine saturated with ammonia is introduced into the carbonating tank from top. Carbonating tower is fitted with a number of compound diaphragms each made of a horizontal iron plate. Following reactions take place here:
2NH3 + CO2 + H2O → (NH4)2CO3
(NH4)2CO3 + 2NaCl → Na2CO3 + 2NH4Cl
Na2CO3 + H2O + CO2 → 2NaHCO3
5. Vacuum filter:
The precipitated sodium bicarbonate along with solution of traces of ammonium carbonate and ammonium chloride is passed through rotatory vacuum filter at the bottom of tower where sodium bicarbonate separates leaving behind mother liquor containing ammonium chloride.
6. Limekiln:
Here limestone is burnt to produce quick lime and carbon dioxide.
CaCO3 \({ \underrightarrow { \Delta } }\) CaO + CO2
Formed CaO reacts with water to form slaked lime.
CaO + H2O → Ca(OH)2
7. Ammonia recovery tower:
The mother liquor obtained from rotatory filter pump containing ammonium chloride is introduced into the ammonia recovery tank from the top part while the milk of lime is introduced from the top of lower part and steam is introduced into the tower from the bottom, ammonium chloride reacts with milk of lime to produce ammonia.
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + 2H2O
Sodium bicarbonate obtained from rotatory filters is ignited in a specially constructed cylindrical vessels when sodium carbonate is formed and CO2 evolved is collected and used again.
2NaHCO3 → Na2CO3 + H2O + CO2
![]()
Question 2.
What happens when:
- Reaction of NaOH with Zn or A1
- Reaction of NaOH with S
- Reaction of NaOH with halogen
- Reaction of NaOH with metal oxides
- Reaction of NaOH with metal salts.
Answer:
1. Zn + 2NaOH → Na2ZnO2 + H2
2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2
2. 4S + 6NaOH → Na2S2O3 + 2Na2S + 3H2O Hypo
8S + 2Na2S → 2Na2S5
3. 2NaOH + Cl2 → NaCl + NaCIO + H2O
(Cold) Sodium hypochlorate
6NaOH + 3Cl2 → 5NaCl + NaClO3 + 3H2O
(Warm)
4. ZnO + 2NaOH → Na2ZnO2 + H2O
5. CuSO4 + 2NaOH → Na2SO4 + Cu(OH)2
3NaOH + FeCl3 → Fe(OH)3 + 3NaCl
2NaOH + 2 AgNO3 → Ag2O + 2NaNO3 + H2O.
Question 3.
How Lithium show similarity with Magnesium?
Answer:
In the periodic table some elements differ from elements of the same period but show diagonal similarity with elements of next period.
Example:
Elements of second period show similarities with elements of third period. Like Lithium (second) period with magnesium (third period), Beryllium with A1 and Boron shows similarity with Si.

The relationship between Li and Mg:
- The Li atomic radius is 1.34 Å and atomic radius of Mg is 1.36 Å .
- The polar capacity of Li and Mg is equal.
- Li and Mg is harder elements.
- The Li and Mg is electronegativity is equal to (1.0 and 1.2).
- The melting and boiling point of Li and Mg is very high.
- Li and Mg reacts with N2 and they form nitrite.
- Li and Mg reacts with O2 and they form monoxide.
- Li and Mg reacts with water to release of hydrogen gas.
- Li and Mg carbonate is heated and release CO2 gas.
- LiOH and Mg(OH)2 is the weak basicity.
Question 4.
Write the formation of calcium oxide and write its properties and uses?
Answer:
Manufacture:
Commercially, calcium oxide is manufactured by heating limestone.
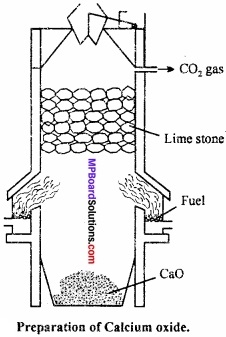
This process is exothermic and reversible. In order to get good yield of lime, carbon dioxide is to be removed from time to time. (MPBoardSolutions.com) The temperature of the reaction should not exceed 900°C because at higher temperature lime and clay reacts to form fusible silicate.

Furnace used for the manufacture of lime contain two fire boxes, one each on each side. Lime stone is added from the top. It decomposes on reach¬ing down. CO2 produced is collected and stored in cylinders in liquid state. Lime farmed gets collected at the bottom of the furnace.
Physical properties:
- It is a white solid.
- Its melting point is high i.e., 2870K.
- It is highly stable and it does not decompose in oxy-hydrogen flame but produces a brilliant white light called lime light.
Chemical properties:
1. It reacts with moist air absorbing CO2 to form Ca(OH)2 and CaCO3
CaO + H2O → Ca(OH)2 (Slaked lime)
CaO + CO2 → CaCO3 (Lime stone)
2. When heated with ammonium salt, ammonia gas evolved.
2NH4Cl + CaO → CaCl2 + H2O + 2NH3
3. When heated with coke at high temperature, calcium carbide is produced.

4. It forms calcium chloride when chlorine gas is passed over hot and dry calcium oxide.
2CaO + 2Cl2 → 2CaCl2 + O2
5. Calcium oxide is a strong basic oxide. It reacts with acid to form salt and water while, with acidic oxide it forms respective salt.
CaO + 2HCl → CaCl2 + H2O
CaO + H2SO4 → CaSO4 + H2O
CaO + CO2 → CaCO3 (Calcium carbonate)
CaO + SiO2 → CaSiO3 (Calcium silicate)
CaO + SO2 → CaSO3 (Calcium sulphite)
3CaO + P2O5 → Ca3(PO4)2 (Calcium phosphate)
6. It forms slaked lime when dissolved in water. It is an exothermic reaction.
CaO + H2O → Ca(OH)2 + 15,000 cal.
Uses:
- For making basic lining in furnaces.
- For drying alcohol and gases.
- Lime water is used as laboratory reagent and in medicine.
- For purification of coal gas and in paper industry.
- As flux in metallurgical process.
- For producing lime light.
- For manufacture of ammonia, sodalime, calcium carbide, cement and glass.
Question 5.
Differentiate between Alkali metals and Alkaline earth metals?
Answer:
Differences between Alkali metals and Alkaline earth metals:
Alkali metals:
- They show +1 oxidation state.
- Their hydroxides are strong bases.
- Their carabonate, sulphate and phosphate compounds of alkaline earth plate are soluble in water.
- Their ionisation energy is comparatively less.
- They are melleable, ductile and lustrous.
Alkaline earth metals:
- These show +2 oxidation state.
- Their hydroxides are comparatively weaker bases.
- These compounds of alkaline earth metals are insoluble in water.
- Their ionization energy is high.
- These are comparatively less.
![]()
Question 6.
Explain the Castner – Kellner cell with diagram to obtained sodium hydroxide?
Answer:
Castner – Kellner Cell:
It consists of rectangular iron tank which is fitted with three slate portion which do not touch the bottom but fit into grooves at bottom. The bottom of cell is covered with mercury and this is brought into circulation with the help of an eccentric wheel. (MPBoardSolutions.com) The outer compartmentA is filled with brine and inner Graphite compartment B with caustic soda solution. Two slot graphite electrode project from the ceiling of the outer compartment of the vessel and act anode. The iron cathode consisting of several rods is filled in middle compartment.
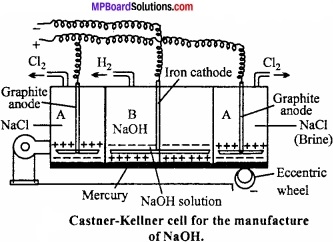
There are exit pipes fitted in compartment and for removal of hydrogen in central compartment. On passing current sodium chloride (brine) solution is electrolyzed in the two outer compartment. Chlorine is liberated at anode. (MPBoardSolutions.com) Sodium amalgam formed is pushed by the help of eccentric wheel into the central compartment. In the central compartment B when caustic soda is electrolyzed OH– move to the mercury layer which act as anode and after discharge react with the sodium of Na – Hg to form caustic soda with the release of H2 gas. Simultaneously an equivalent amount of sodium liberated at cathode reacts with water to produce more caustic soda.
2Na – Hg + 2H2O → 2NaOH + 2Hg + H2↑
The mercury obtained can again be used in the cell. Caustic soda solution is evaporated to obtain solid caustic soda which is fused and cast into flakes or sticks.
Cell reactions are as follows:
1. In outer compartments:
Ionization:
2 NaCl ⇄ 2Na+ + 2Cl–; (Ionization)
At cathode:
2Na+ + 2e– → 2Na (Reduction)
2Na + xHg → HgxNa2 (Sodium amalgam)
At anode:
2Cl– → 2Cl + 2e–; (Oxidation)
2Cl → Cl2-
2. In central compartment:
Ionization:
NaOH ⇄ Na+ + OH–; (Ionization)
At cathode:
2Na – Hg + 2H20 2NaOH + 2Hg + H2
Na+ + e– → Na, (Reduction)
2Na +2H2O 2NaOH + H2(g)
At anode:
2OH– → 20H + 2e–, (Oxidation)
HgxNa2 + 20H → 2NaOH + xHg